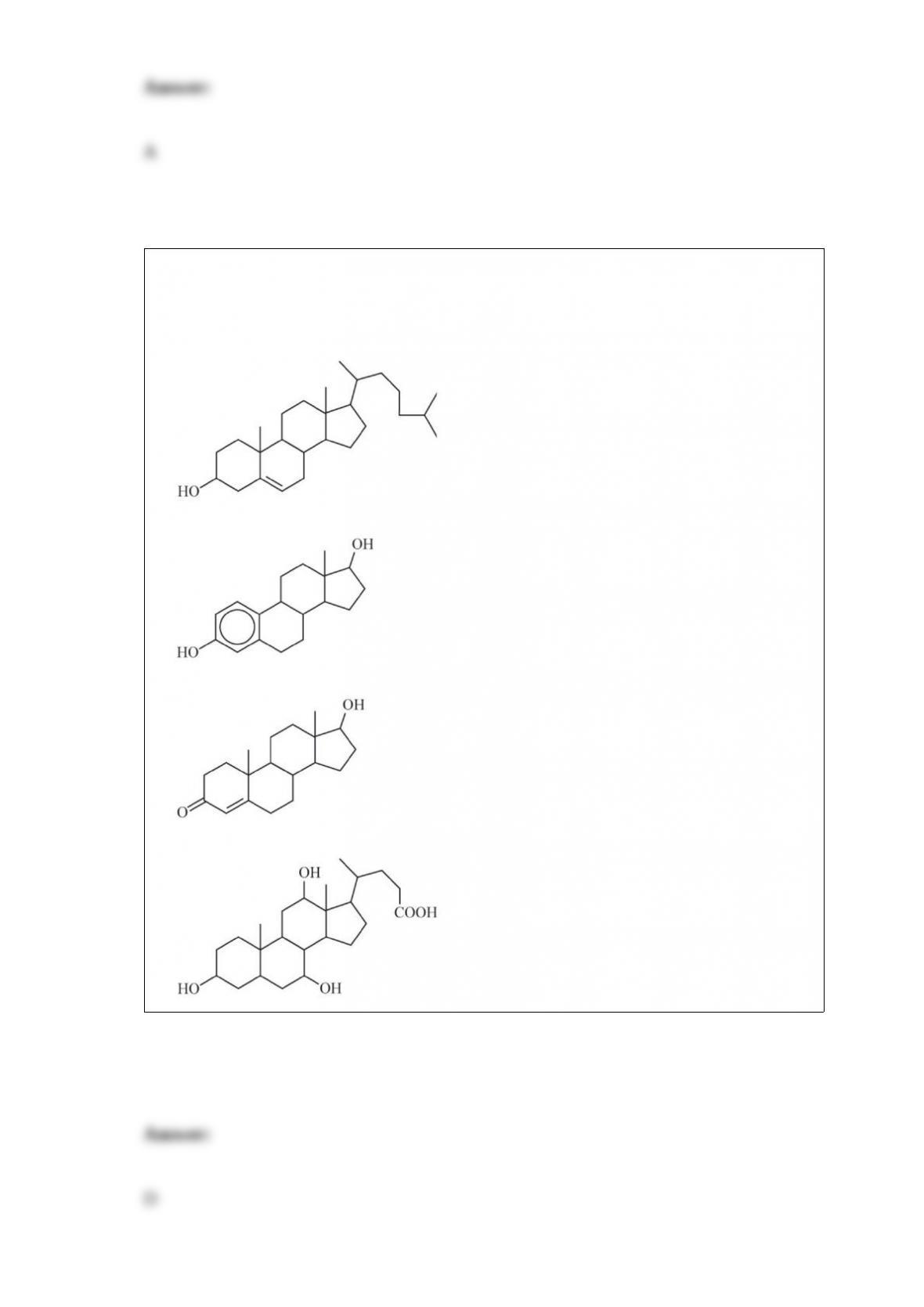
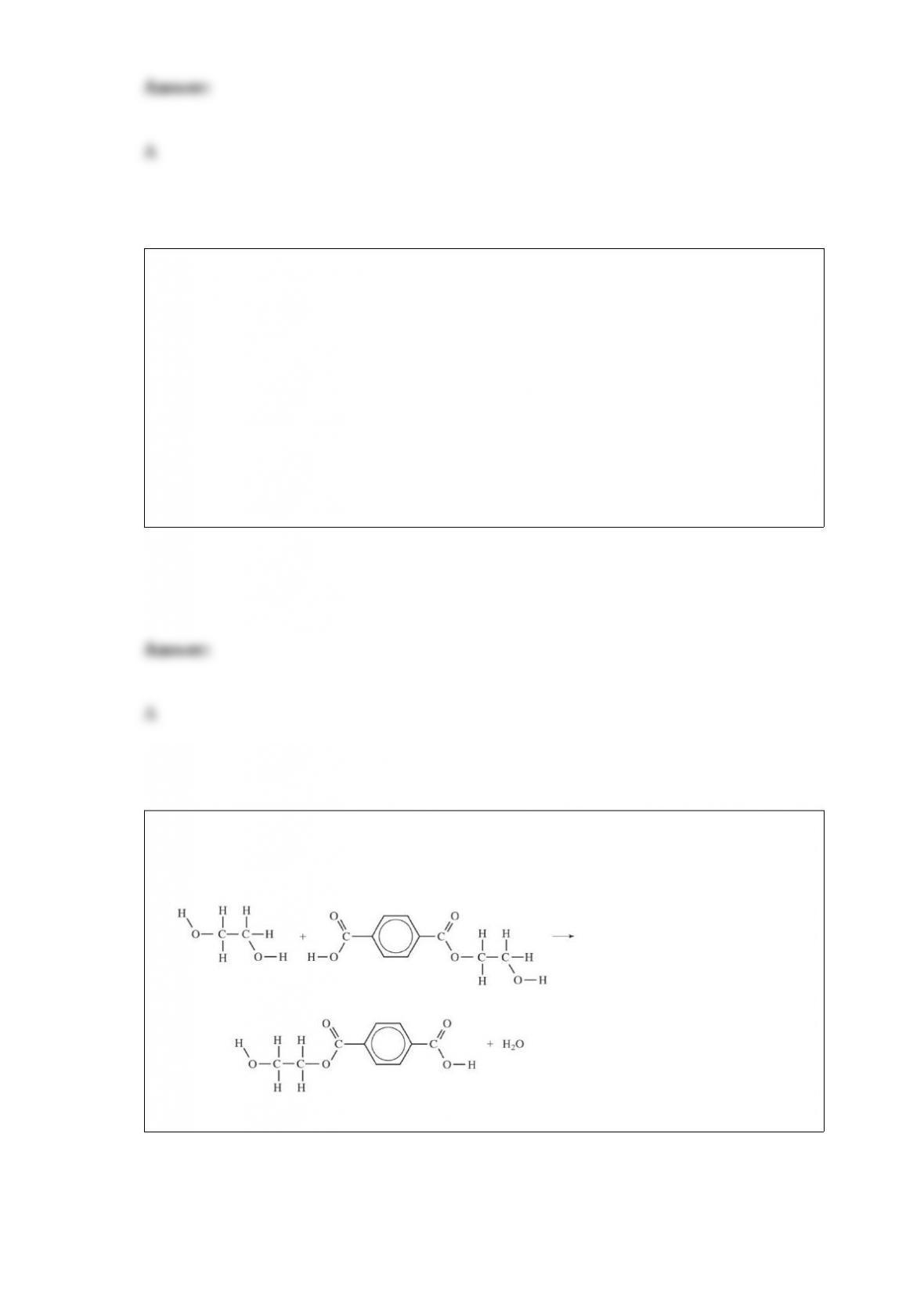
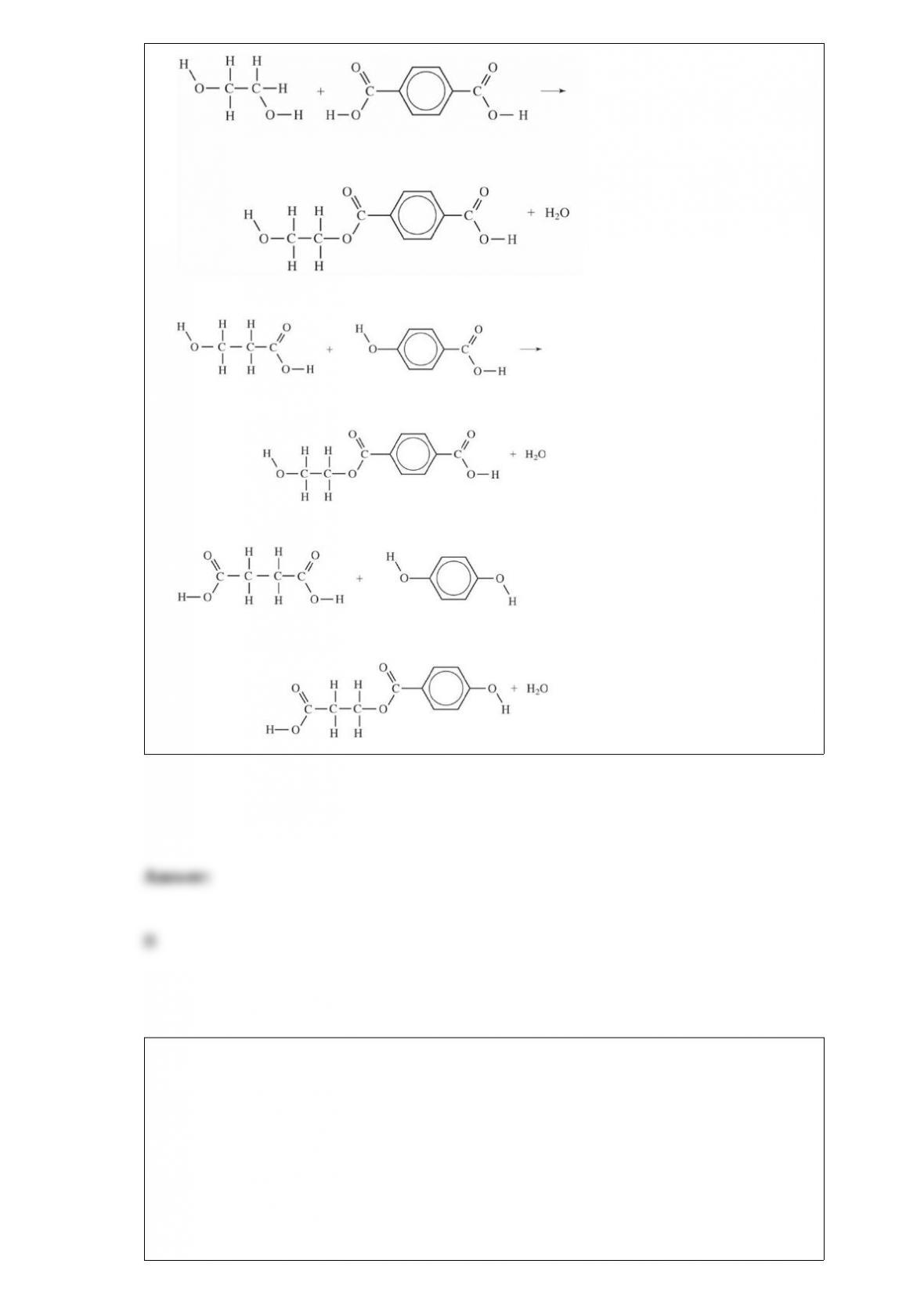
B. carbon dioxide
C. nitrogen
D. argon
In a solar cell, semiconductors of the p-type and n-type are placed in contact with each
other via a conducting wire. In order to generate an electric current, which of the
following must be true?22-2
A. An external battery must be attached
B. Light shining on the system will cause oxidation to occur
C. Light shining on the system must have enough energy to set electrons in motion from
the n-type semiconductors to the p-type
D. Light shining on the system must have enough energy to set electrons in motion
from the p-type semiconductors to the n-type
Why are HFCs environmentally superior to the currently used HCFCs?
A. HFCs are not flammable.
B. HFCs do not contain chlorine.