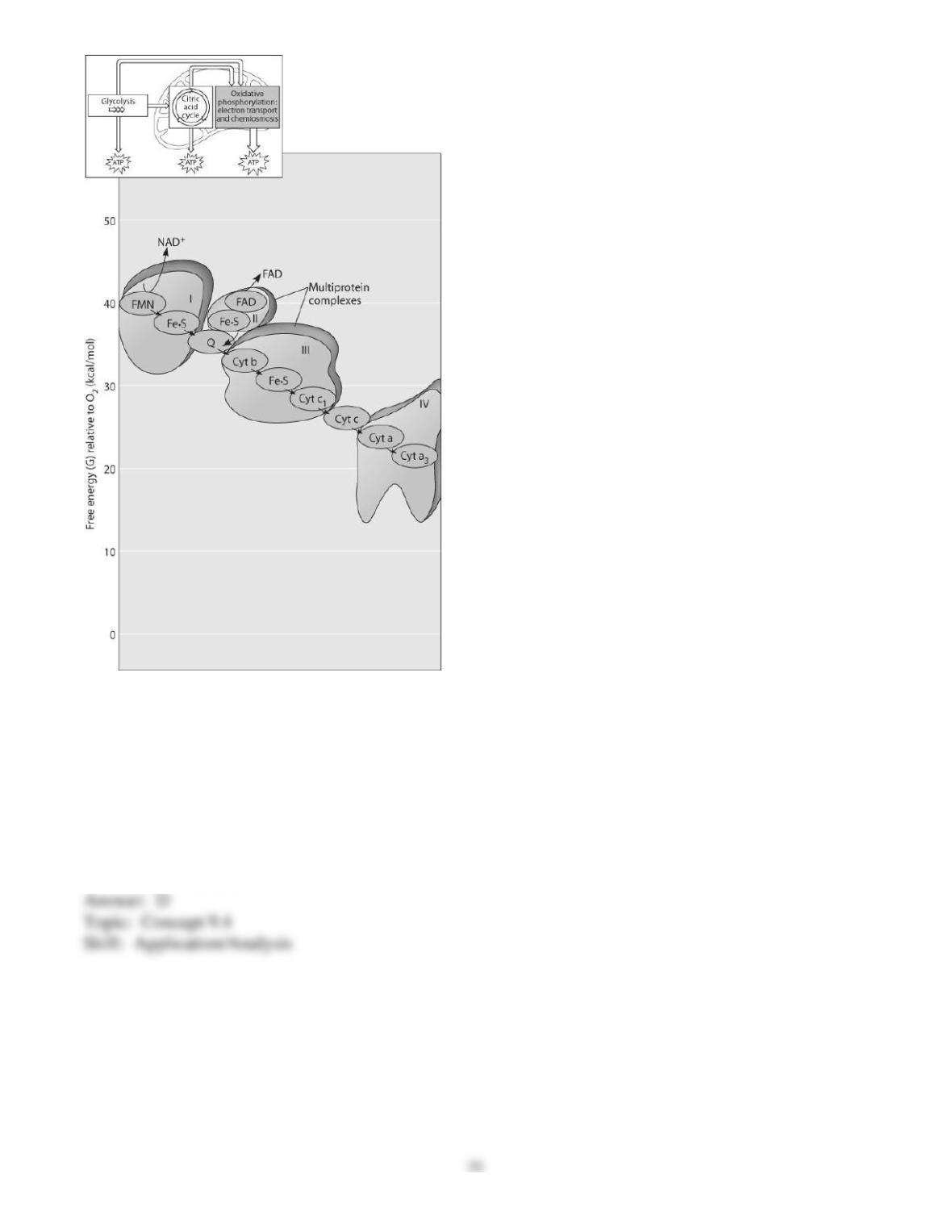
70) You have a friend who lost 7 kg (about 15 pounds) of fat on a regimen of strict diet and exercise.
How did the fat leave her body?
A) It was released as CO2 and H2O.
B) It was converted to heat and then released.
C) It was converted to ATP, which weighs much less than fat.
D) It was broken down to amino acids and eliminated from the body.
E) It was converted to urine and eliminated from the body.
71) Phosphofructokinase is an important control enzyme in the regulation of cellular respiration. Which
of the following statements correctly describes phosphofructokinase activity?
A) It is inhibited by AMP.
B) It is activated by ATP.
C) It is activated by citrate, an intermediate of the citric acid cycle.
D) It catalyzes the conversion of fructose 1,6-bisphosphate to fructose 6-phosphate, an early step of
glycolysis.
E) It is an allosteric enzyme.
72) Phosphofructokinase is an allosteric enzyme that catalyzes the conversion of fructose 6-phosphate to
fructose 1,6-bisphosphate, an early step of glycolysis. In the presence of oxygen, an increase in the
amount of ATP in a cell would be expected to
A) inhibit the enzyme and thus slow the rates of glycolysis and the citric acid cycle.
B) activate the enzyme and thus slow the rates of glycolysis and the citric acid cycle.
C) inhibit the enzyme and thus increase the rates of glycolysis and the citric acid cycle.
D) activate the enzyme and increase the rates of glycolysis and the citric acid cycle.
E) inhibit the enzyme and thus increase the rate of glycolysis and the concentration of citrate.
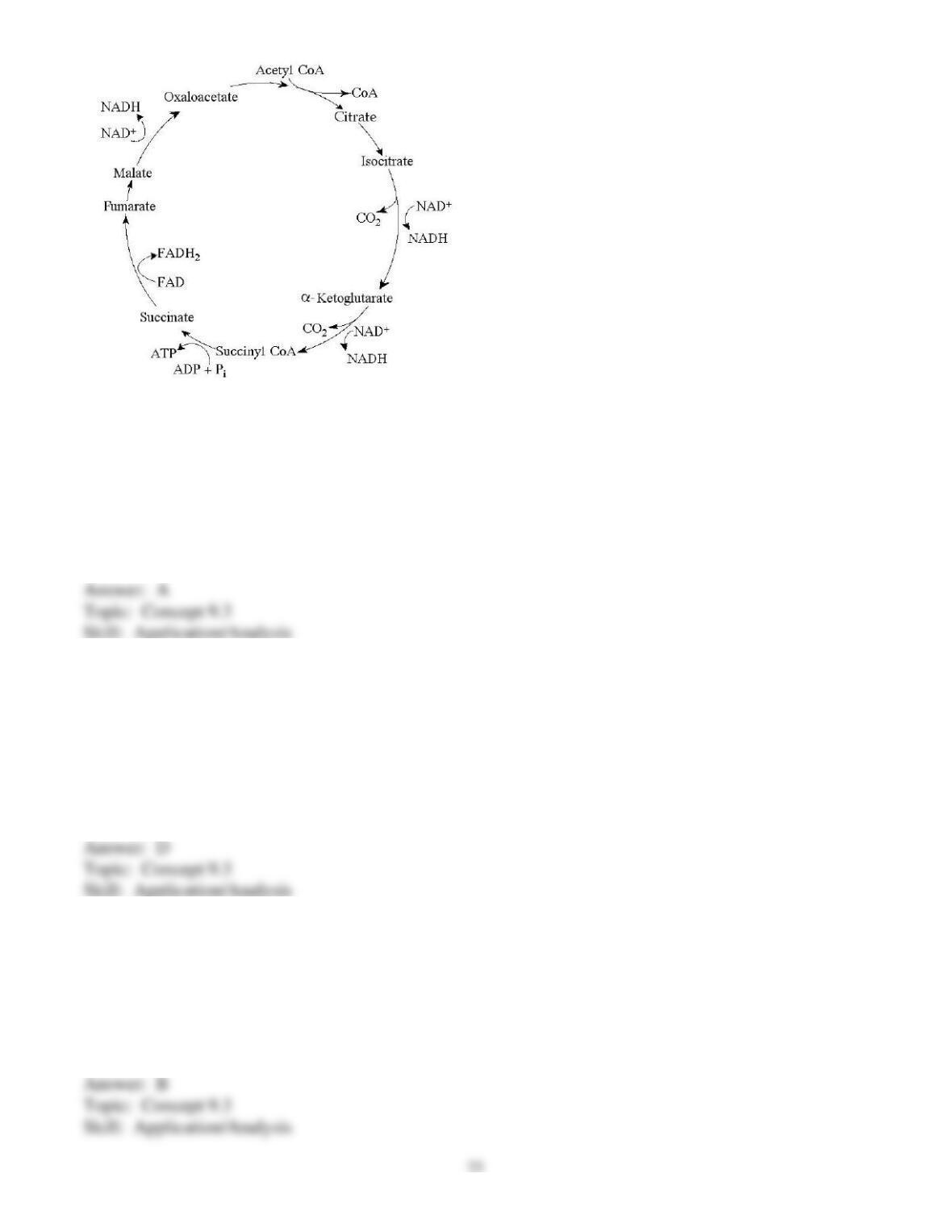
73) Even though plants carry on photosynthesis, plant cells still use their mitochondria for oxidation of
pyruvate. When and where will this occur?
A) in photosynthetic cells in the light, while photosynthesis occurs concurrently
B) in nonphotosynthesizing cells only
C) in cells that are storing glucose only
D) in all cells all the time
E) in photosynthesizing cells in the light and in other tissues in the dark