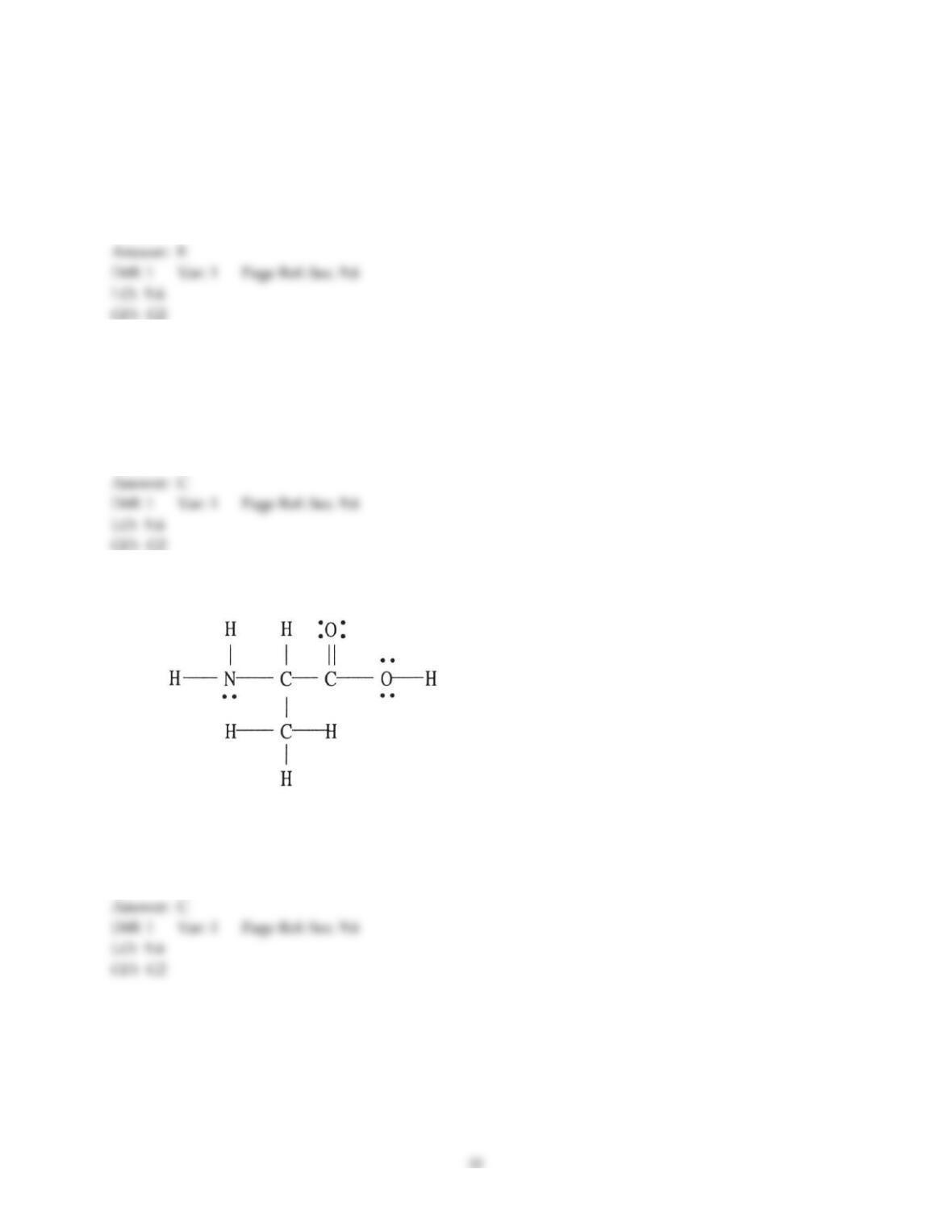
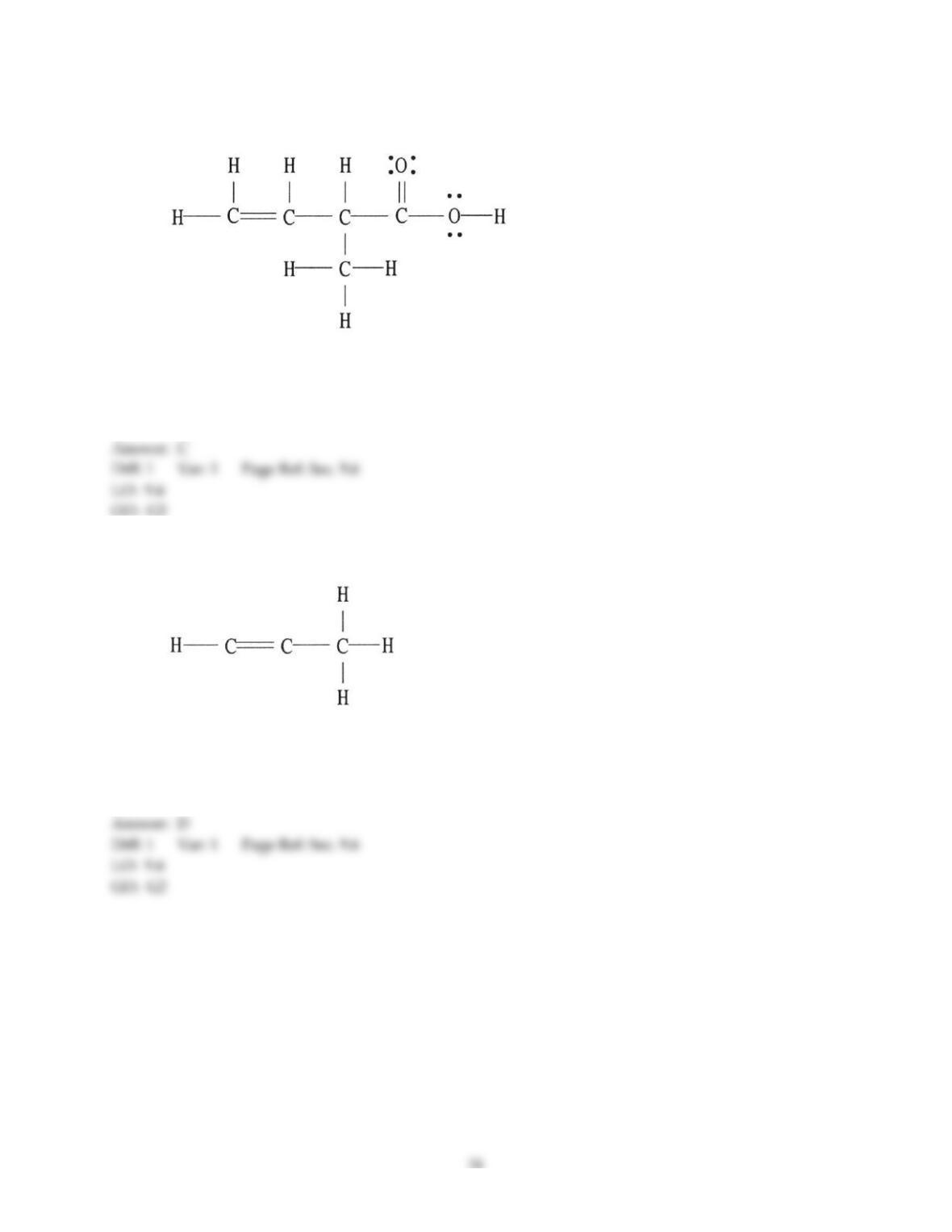
26
87) The bond order of a homonuclear diatomic molecule can be decreased by ________.
A) removing electrons from a bonding MO or adding electrons to an antibonding MO
B) adding electrons to a bonding MO or removing electrons from an antibonding MO
C) adding electrons to any MO
D) removing electrons from any MO
E) The bond order of a homonuclear diatomic molecule cannot be decreased by any means.
88) The order of MO energies in B2, C2, and N2 (σ2p > π2p), is different from the order in O2, F2, and
Ne2 (σ2p > π2p). This is due to ________.
A) less effective overlap of p orbitals in O2, F2, and Ne2
B) the more metallic character of boron, carbon and nitrogen as compared to oxygen, fluorine, and neon
C) greater 2s-2p interaction in O2, F2, and Ne2
D) greater 2s-2p interaction in B2, C2, and N2
E) less effective overlap of p orbitals in B2, C2, and N2
9.2 Bimodal Questions
1) For a molecule with the formula AB2, the molecular shape is ________.
A) linear or bent
B) linear or trigonal planar
C) linear or T-shaped
D) T-shaped
E) trigonal planar
2) For a molecule with the formula AB3, the molecular shape is ________.
A) linear, octahedral, or bent
B) linear, bent, or trigonal planar
C) linear, bent, or T-shaped
D) tetrahedral
E) trigonal planar, trigonal pyramidal, or T-shaped
3) The molecular geometry of ________ is square planar.