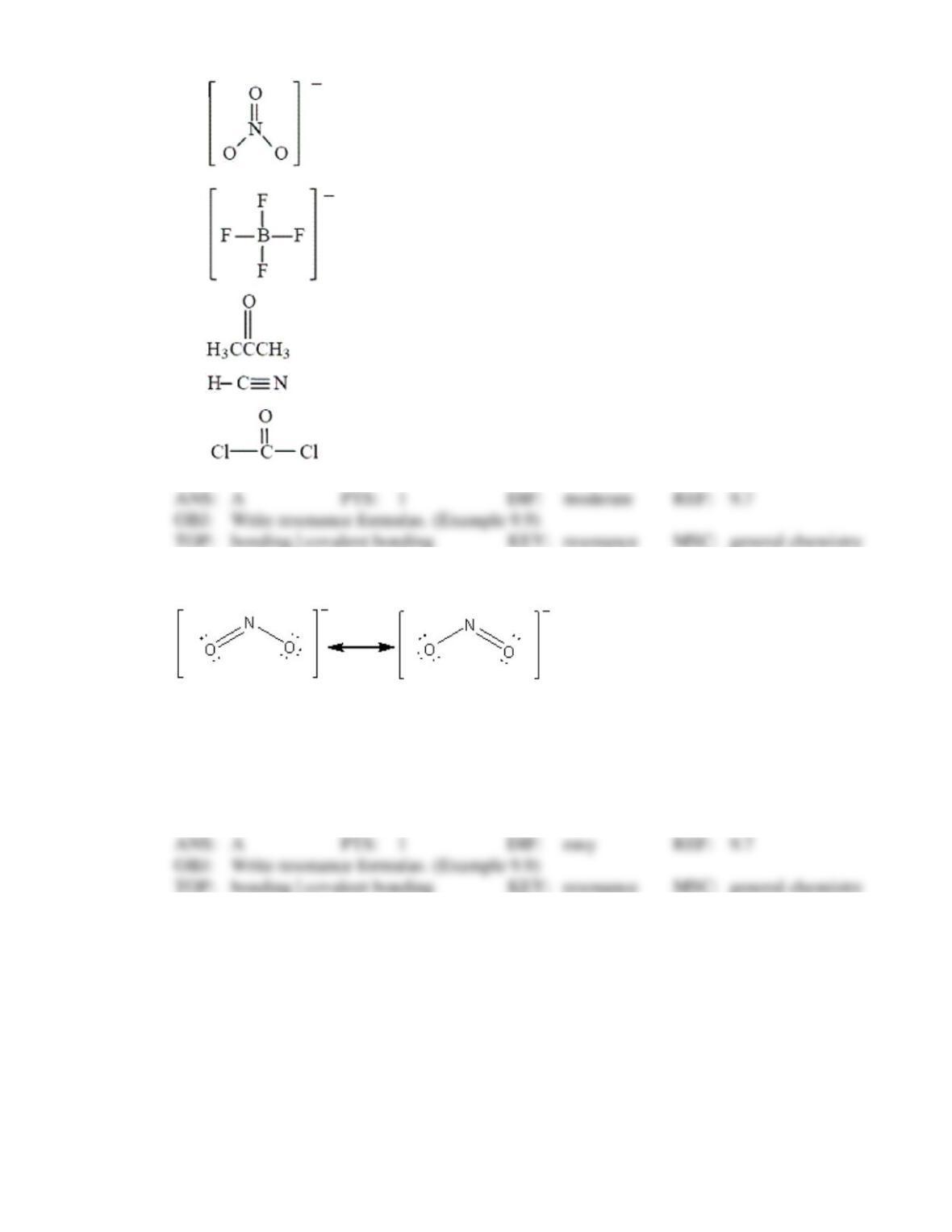
87. In the Lewis formula for hydrazinium ion, N2H5+, the total number of lone electron pairs
around the two nitrogen atoms is
88. In the Lewis formula for the hydroxide ion, OH-, the number of lone pairs of electrons
around the oxygen atom is
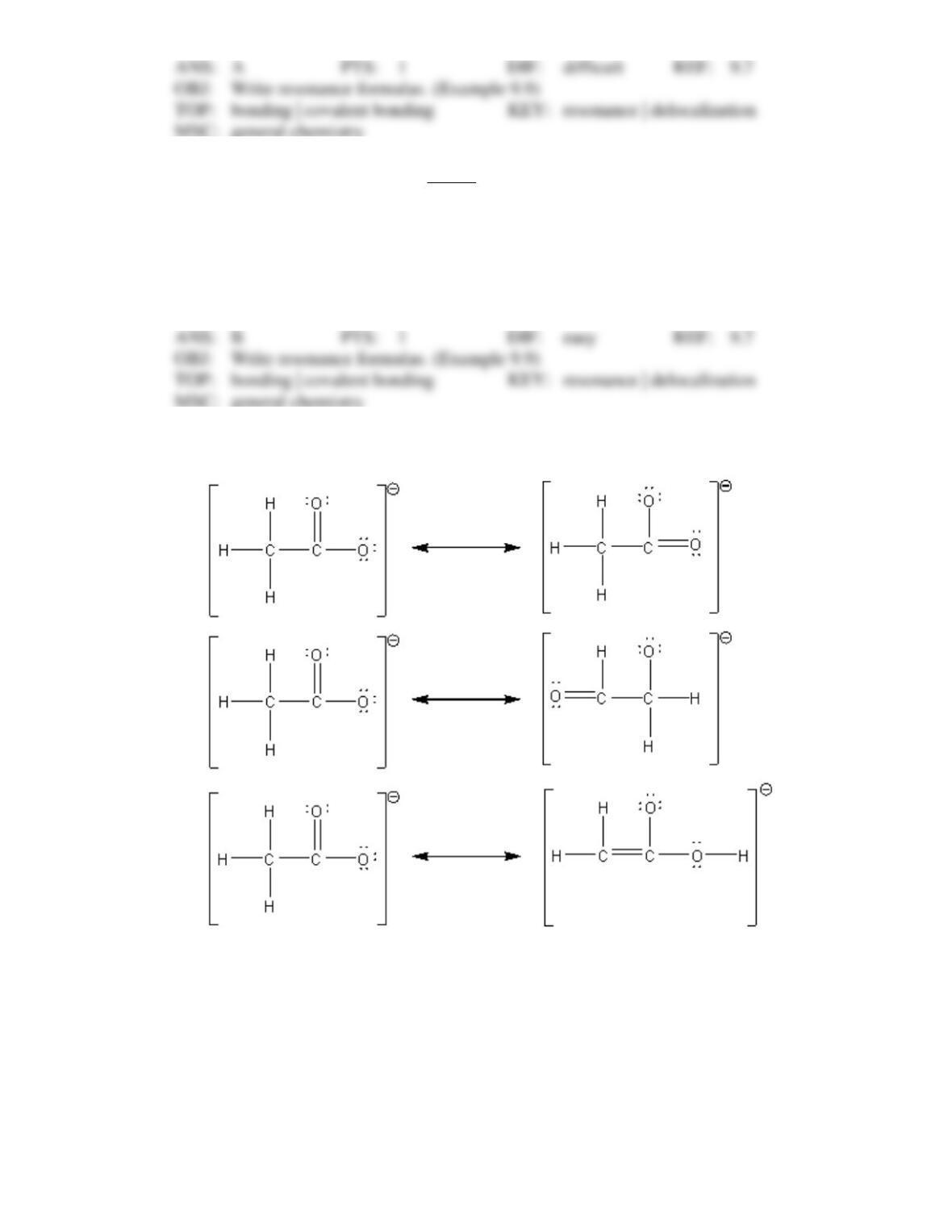
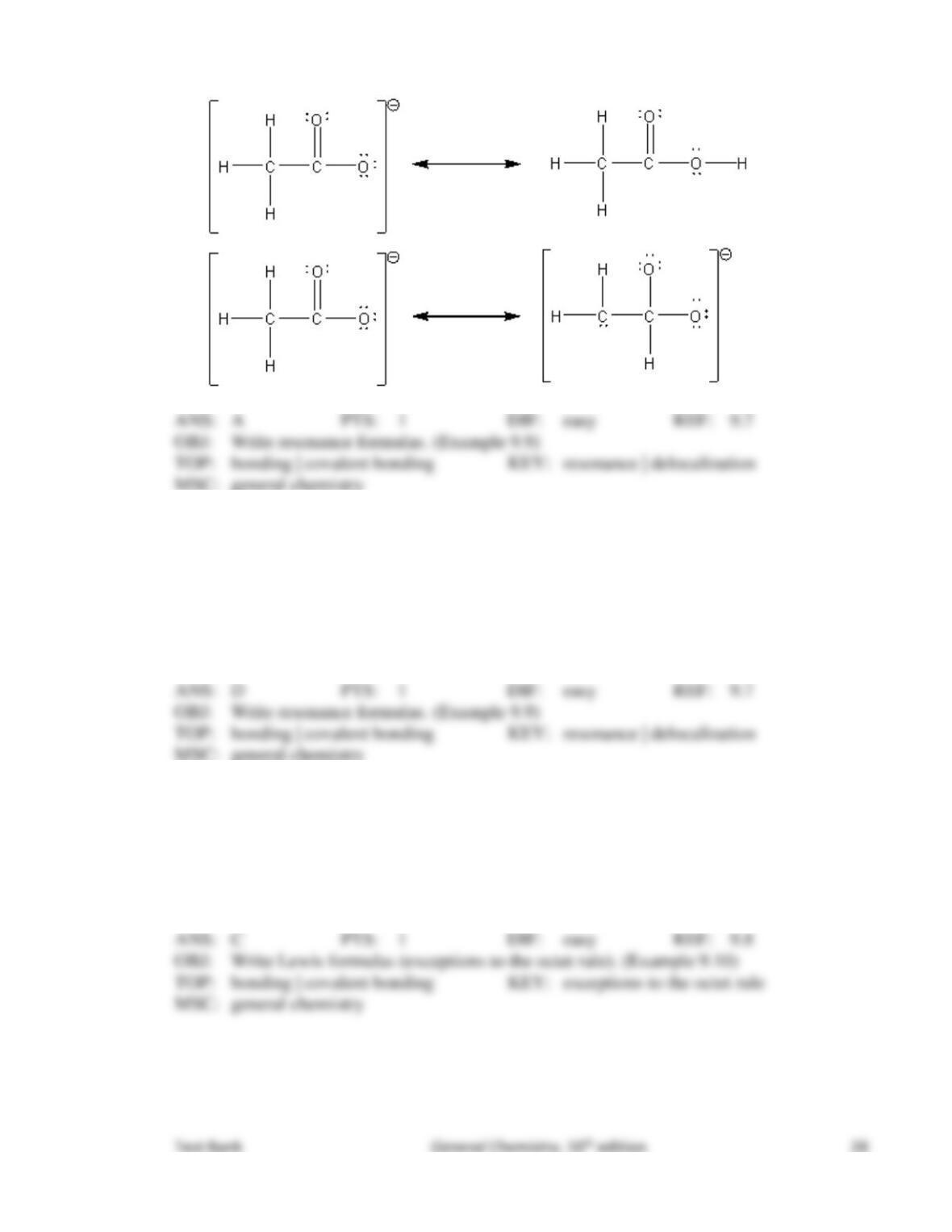
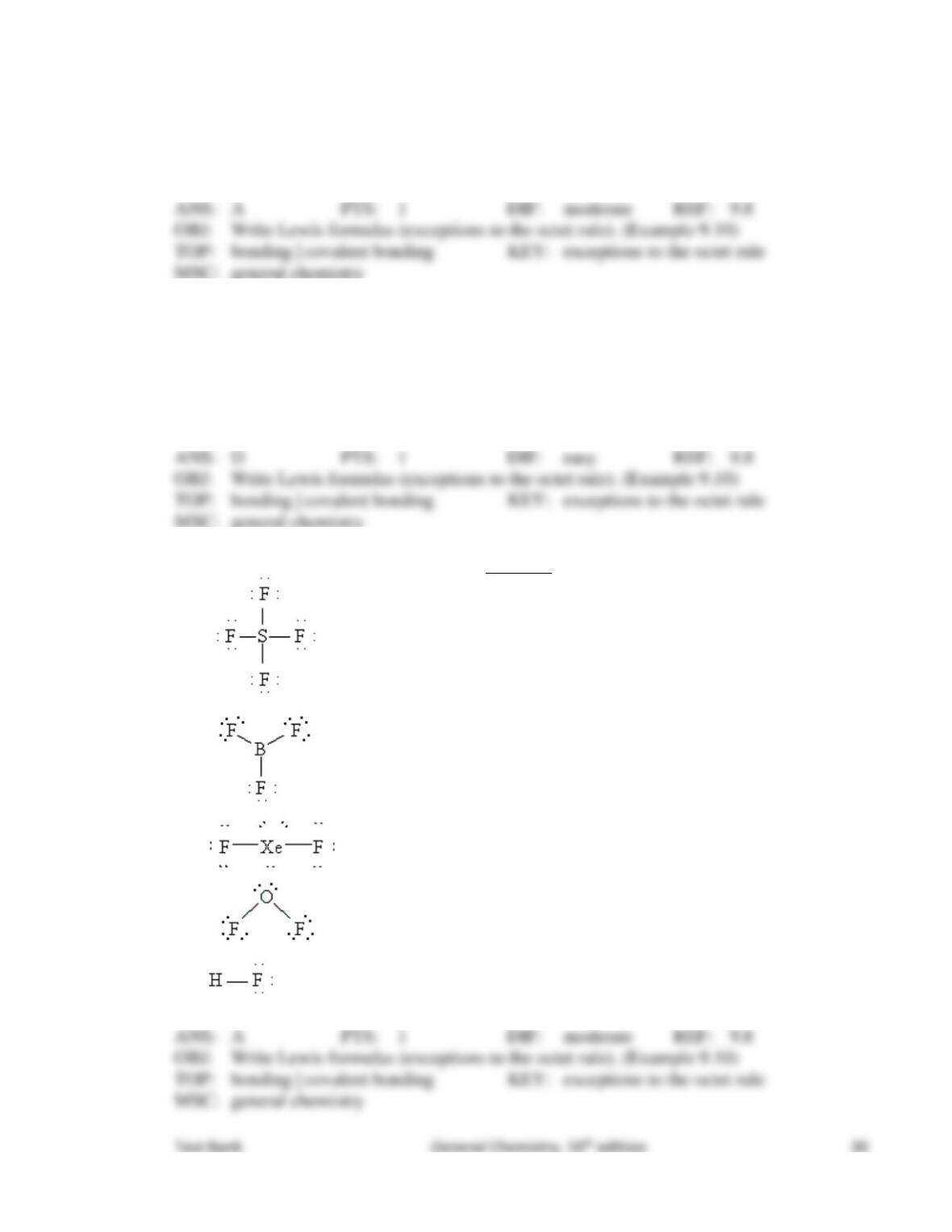
89. The concept of resonance describes molecular structures
that have several different geometric arrangements.
that have delocalized bonding.
that are formed from hybridized orbitals.
that have different molecular formulas.
that have electrons resonating.
90. All the following statements about resonance are true except
A single Lewis formula does not provide an adequate representation of the
bonding.
Resonance describes a more stable situation than does any one contributing
resonance formula.
Resonance describes the oscillation and vibration of electrons.
The contributing resonance formulas differ only in the arrangement of the
electrons.
Resonance describes the bonding as intermediate between the contributing
resonance formulas.