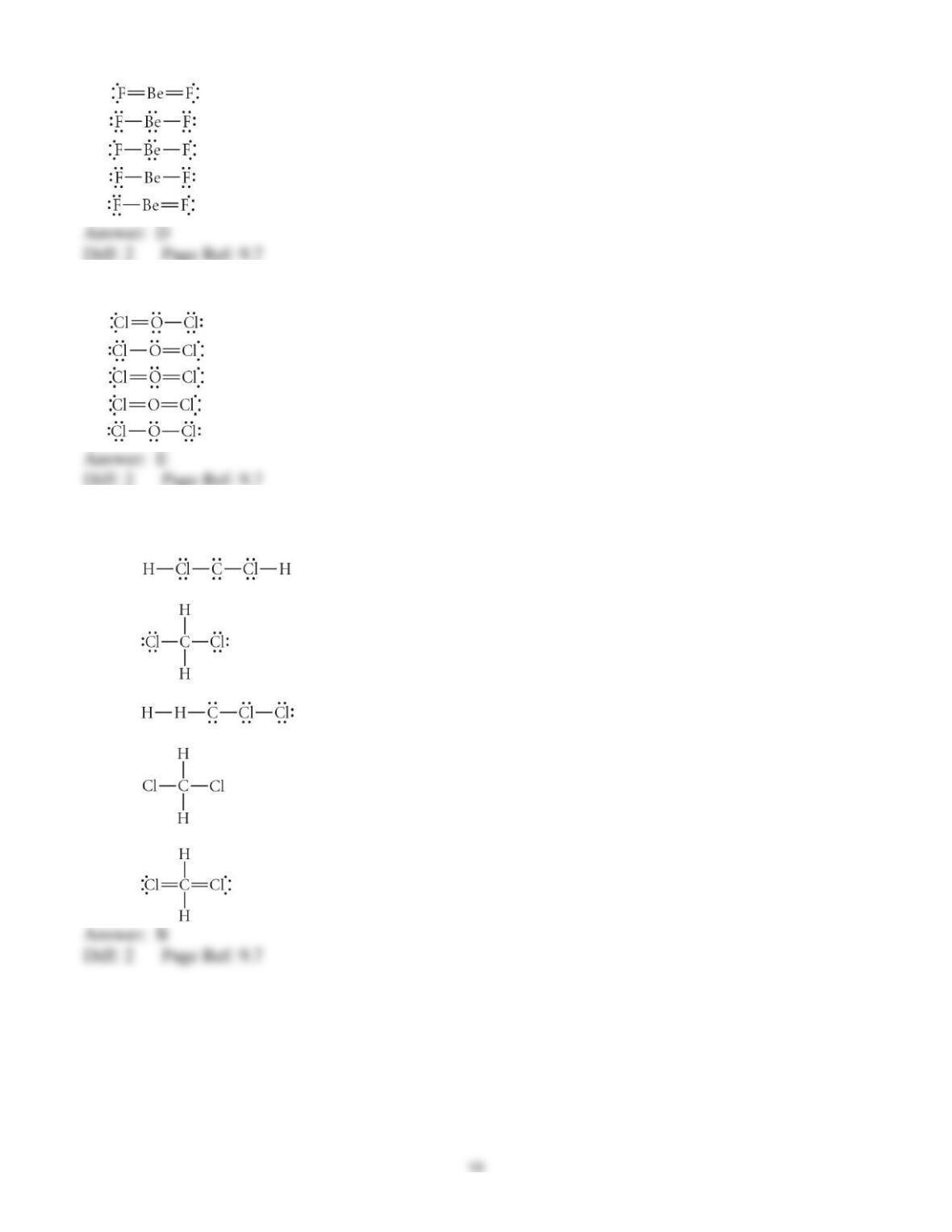
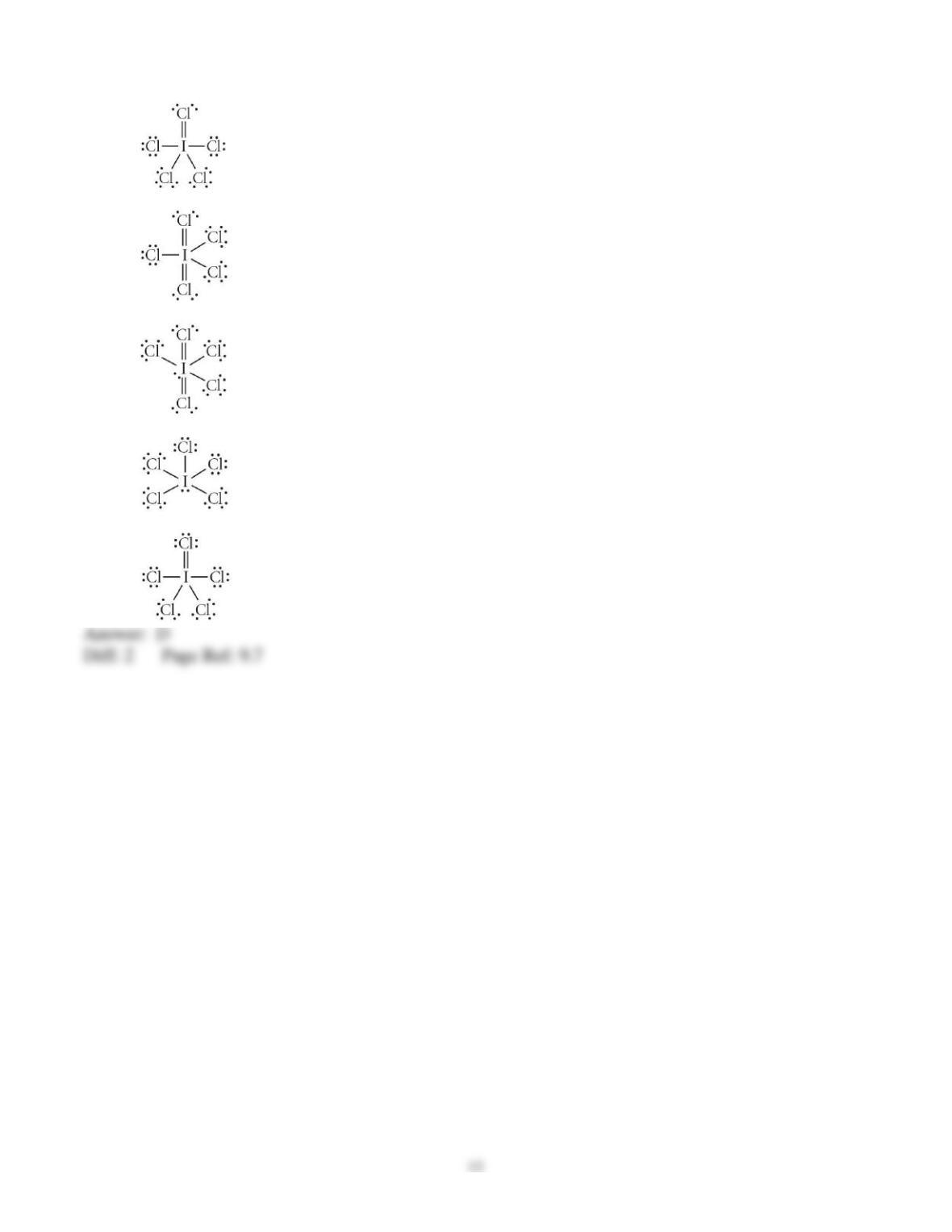
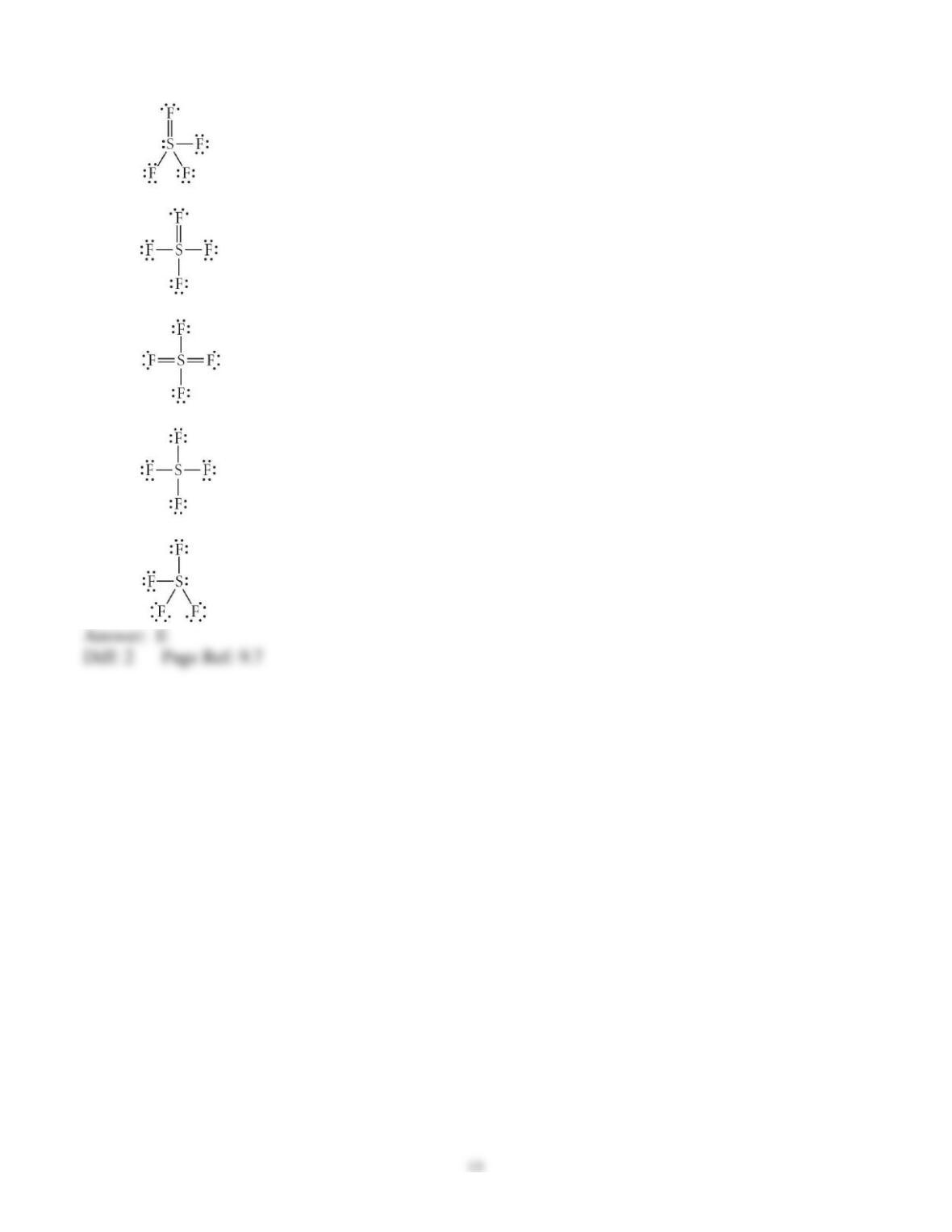
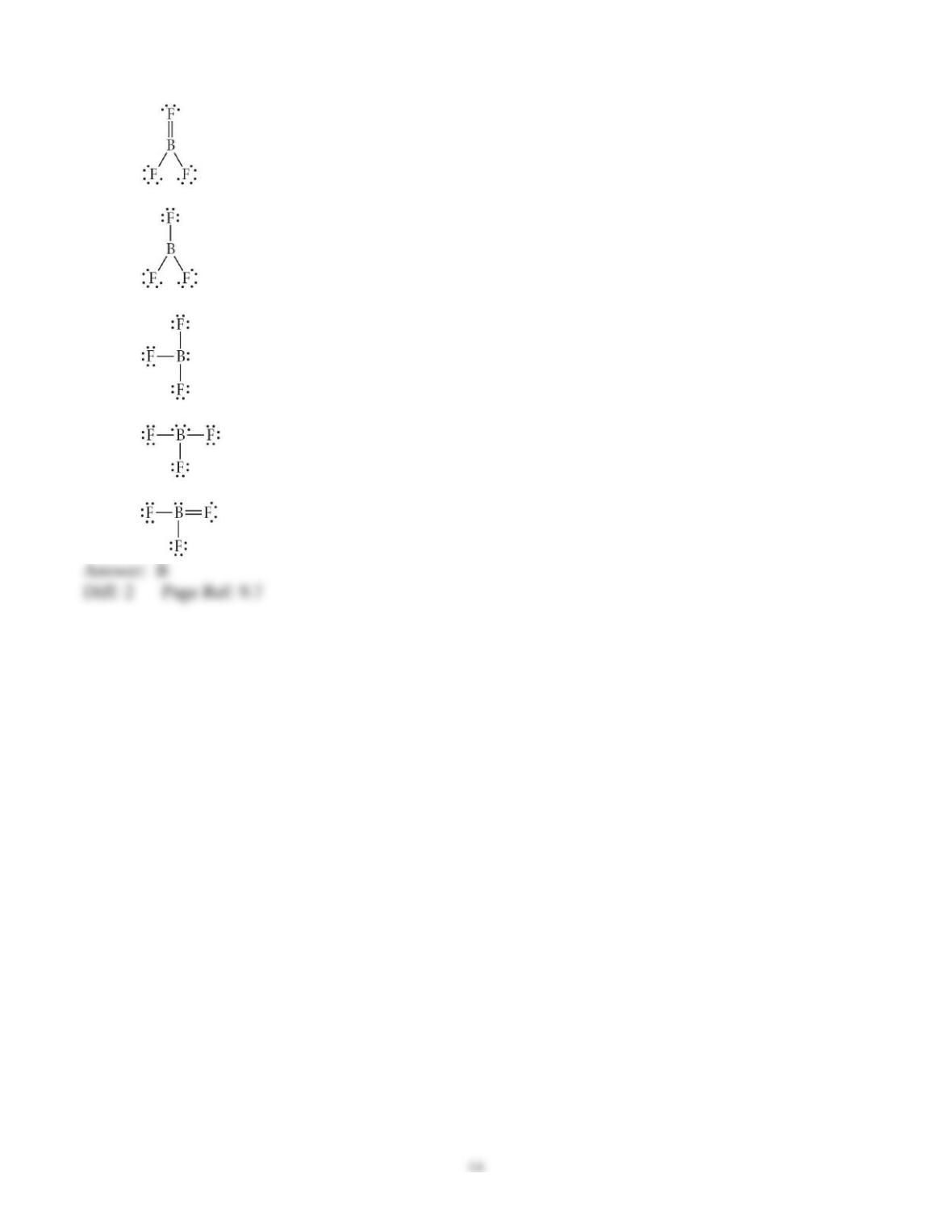
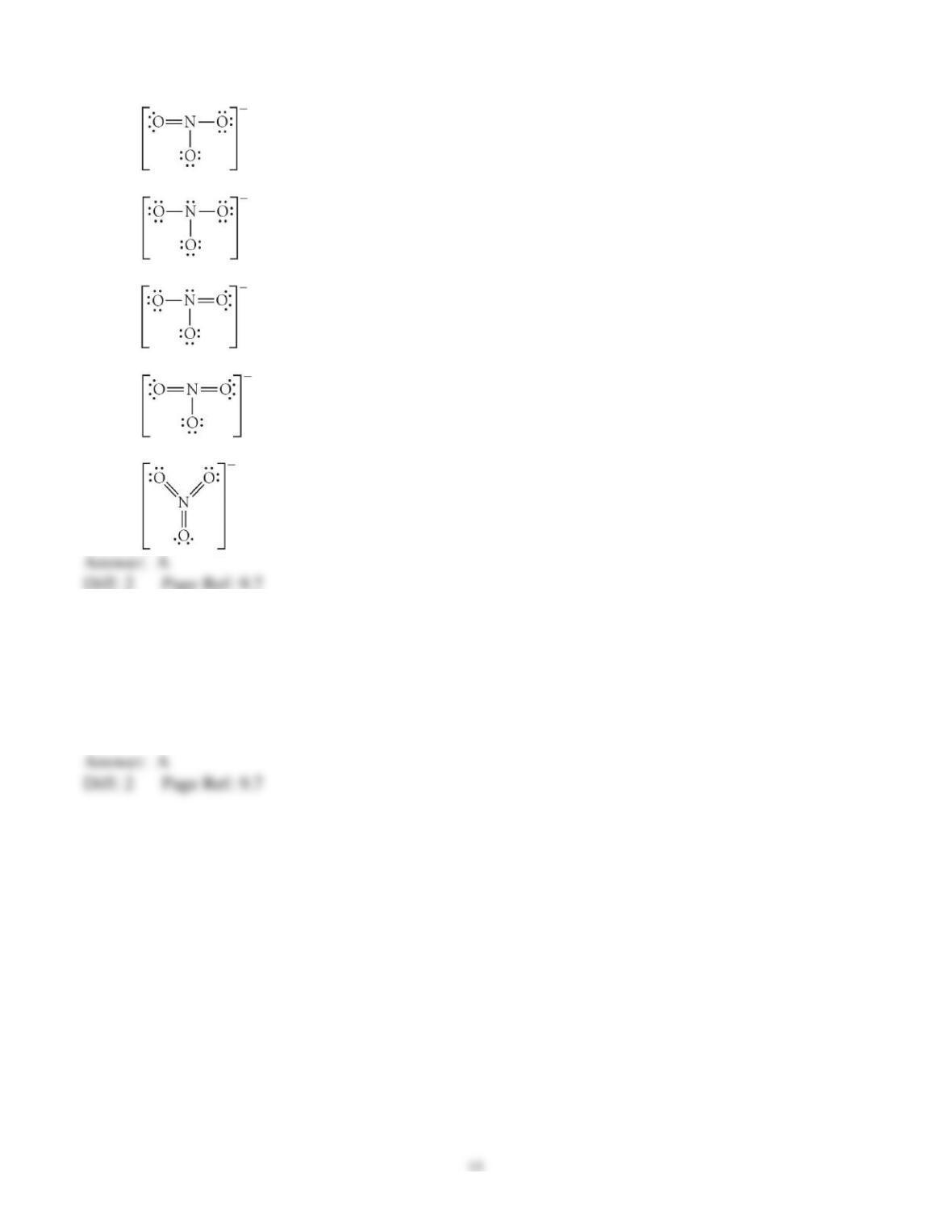
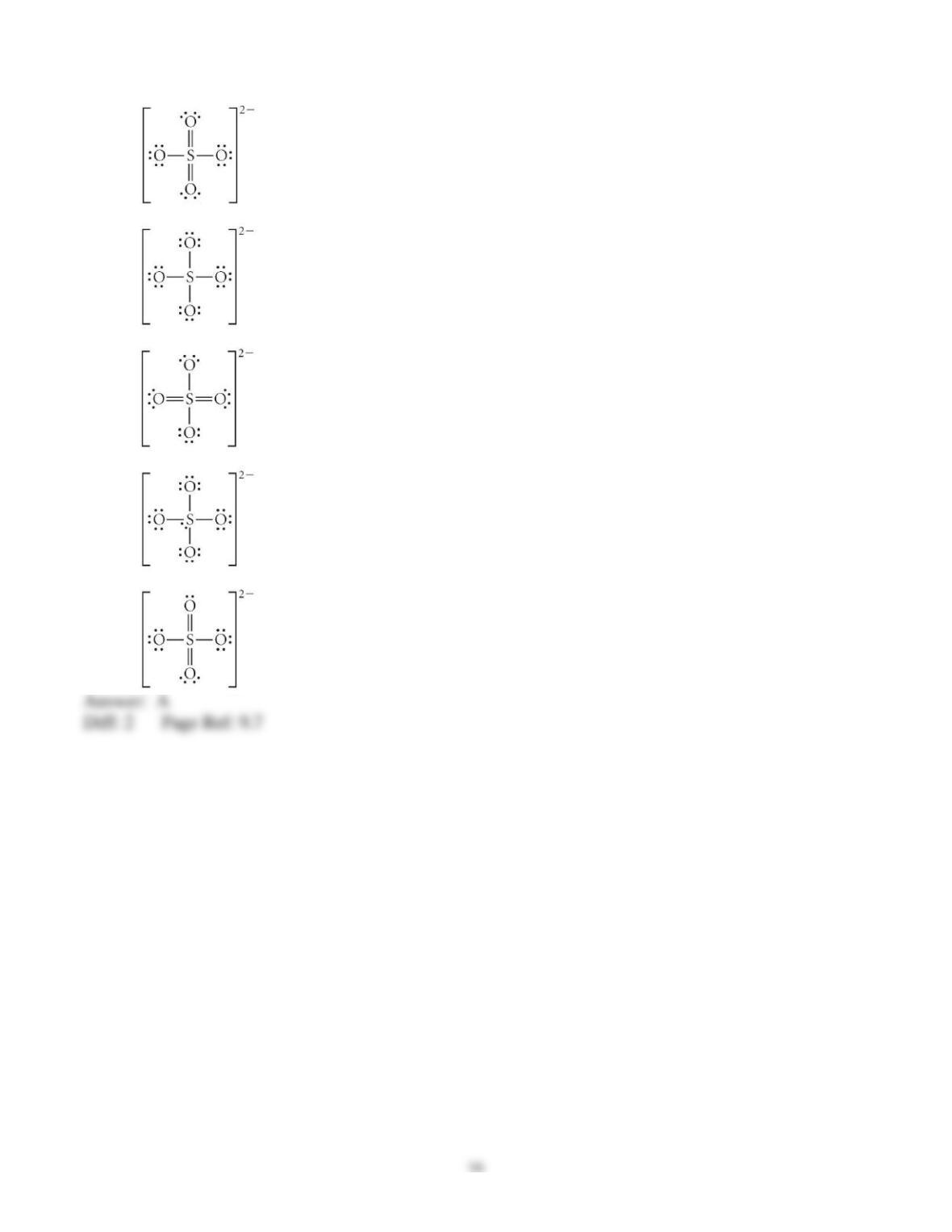
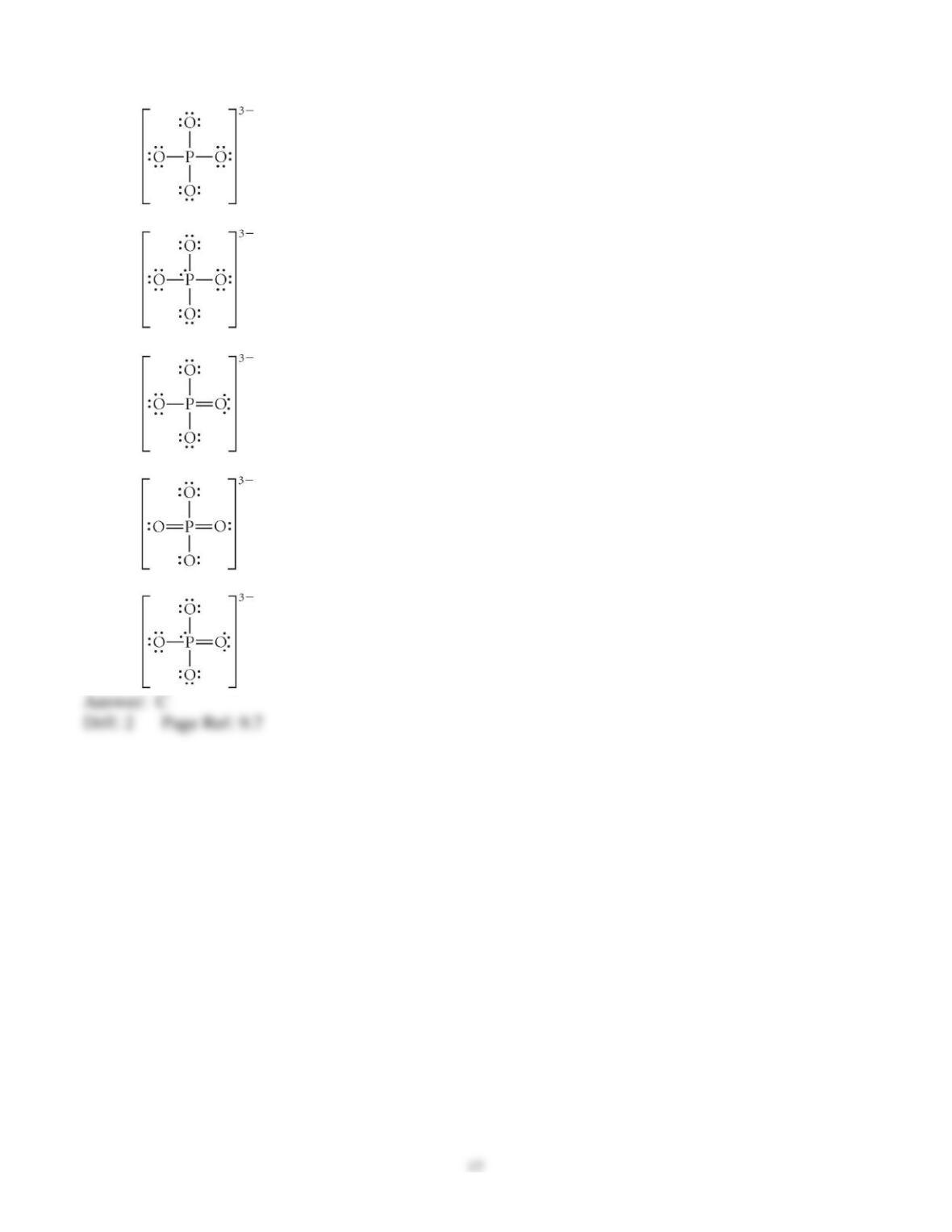
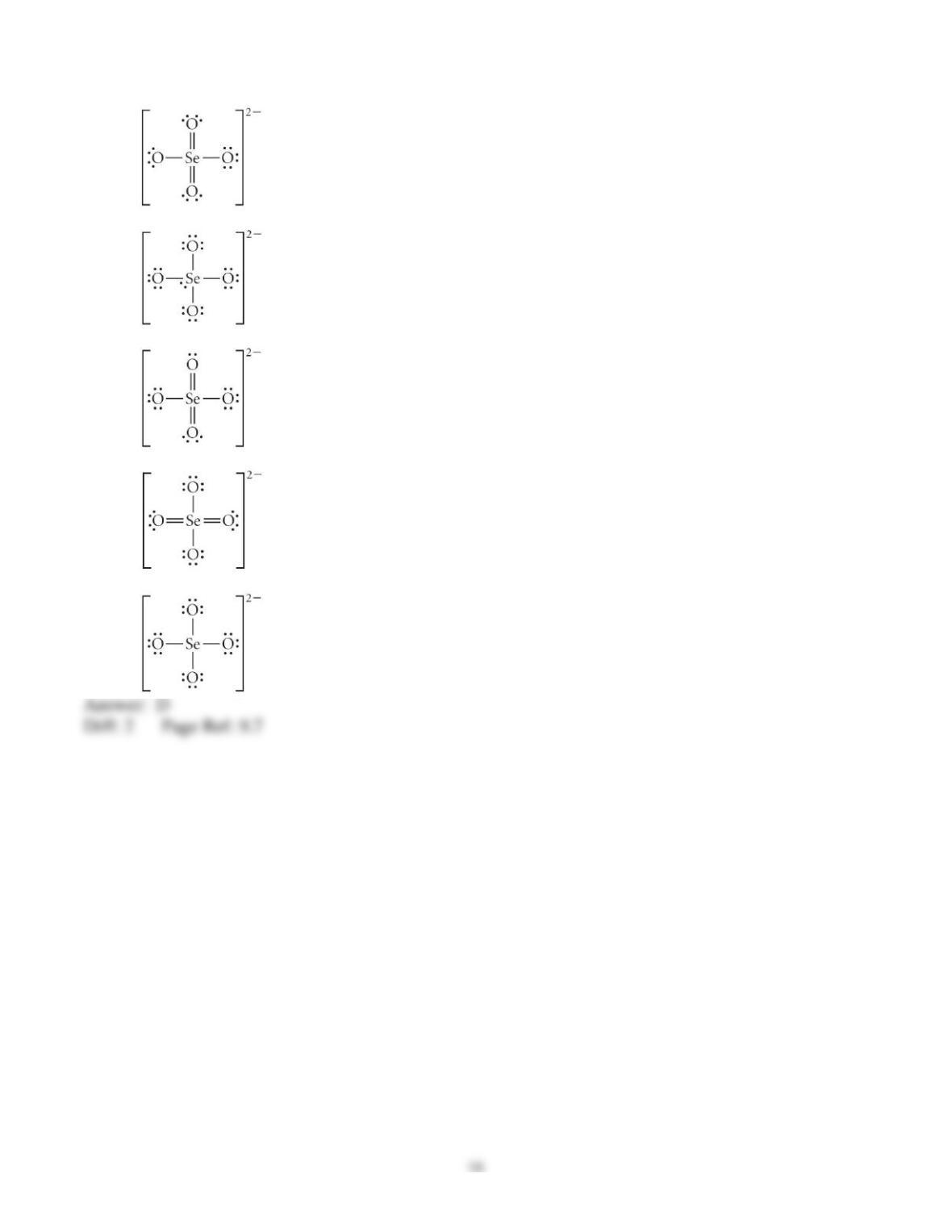
3
A) 1s22s22p63s24p6
B) 1s22s22p63s23p6
C) 1s22s22p63s23p5
D) 1s22s23p64s25p6
E) 1s22s2p63s2p6
11) Which of the following statements is TRUE?
A) An ionic bond is much stronger than most covalent bonds.
B) An ionic bond is formed through the sharing of electrons.
C) Ionic compounds at room temperature typically conduct electricity.
D) Once dissolved in water, ionic compounds rarely conduct electricity.
E) None of the above are true.
12) Use Lewis theory to determine the chemical formula for the compound formed between Al and O.
A) Al3O2
B) Al2O3
C) AlO2
D) Al2O
E) AlO
13) Which of the following reactions is associated with the lattice energy of Li2O (ΔH°latt)?
A) Li2O(s) → 2 Li⁺(g) + O2⁻(g)
B) 2 Li⁺(aq) + O2⁻(aq) → Li2O(s)
C) 2 Li⁺(g) + O2⁻(g) → Li2O(s)
D) Li2O(s) → 2 Li⁺(aq) + O2⁻(aq)
E) 2 Li(s) + O2(g) → Li2O(s)
14) Which of the following reactions is associated with the lattice energy of CaS (ΔH°latt)?
A) Ca(s) + S(s) → CaS(s)
B) CaS(s) → Ca(s) + S(s)
C) Ca2⁺(aq) + S2⁻(aq) → CaS(s)
D) Ca2⁺(g) + S2⁻(g) → CaS(s)
E) CaS(s) → Ca2⁺(aq) + S2⁻(aq)
15) Which of the following reactions is associated with the lattice energy of RbI (ΔH°latt)?