20
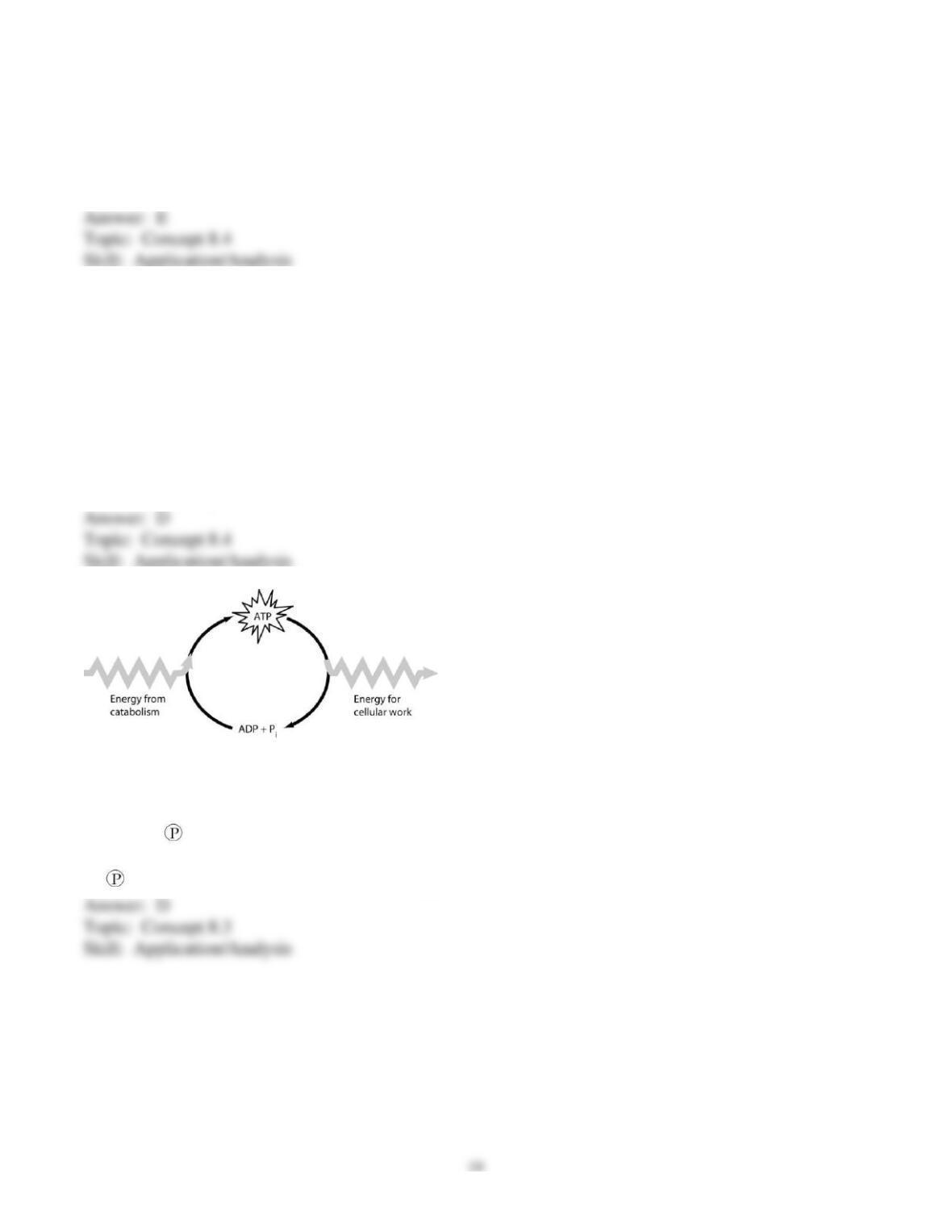
70) How do cells use the ATP cycle shown in the figure?
A) Cells use the cycle to recycle ADP and phosphate.
B) Cells use the cycle to recycle energy released by ATP hydrolysis.
C) Cells use the cycle to recycle ADP, phosphate, and the energy released by ATP hydrolysis.
D) Cells use the cycle to generate or consume water molecules as needed.
E) Cells use the cycle primarily to generate heat.
Scenario Questions
Succinate dehydrogenase catalyzes the conversion of succinate to fumarate. The reaction is inhibited by
malonic acid, which resembles succinate but cannot be acted upon by succinate dehydrogenase.
Increasing the ratio of succinate to malonic acid reduces the inhibitory effect of malonic acid.
71) Based on this information, which of the following is correct?
A) Succinate dehydrogenase is the enzyme, and fumarate is the substrate.
B) Succinate dehydrogenase is the enzyme, and malonic acid is the substrate.
C) Succinate is the substrate, and fumarate is the product.
D) Fumarate is the product, and malonic acid is a noncompetitive inhibitor.
E) Malonic acid is the product, and fumarate is a competitive inhibitor.
72) What is malonic acid's role with respect to succinate dehydrogenase?
A) It is a competitive inhibitor.
B) It blocks the binding of fumarate.
C) It is a noncompetitive inhibitor.
D) It is able to bind to succinate.
E) It is an allosteric regulator.
A series of enzymes catalyze the reaction X → Y → Z → A. Product A binds to the enzyme that
converts X to Y at a position remote from its active site. This binding decreases the activity of the
enzyme.
73) What is substance X?
A) a coenzyme
B) an allosteric inhibitor
C) a substrate
D) an intermediate
E) the product
74) With respect to the enzyme that converts X to Y, substance A functions as