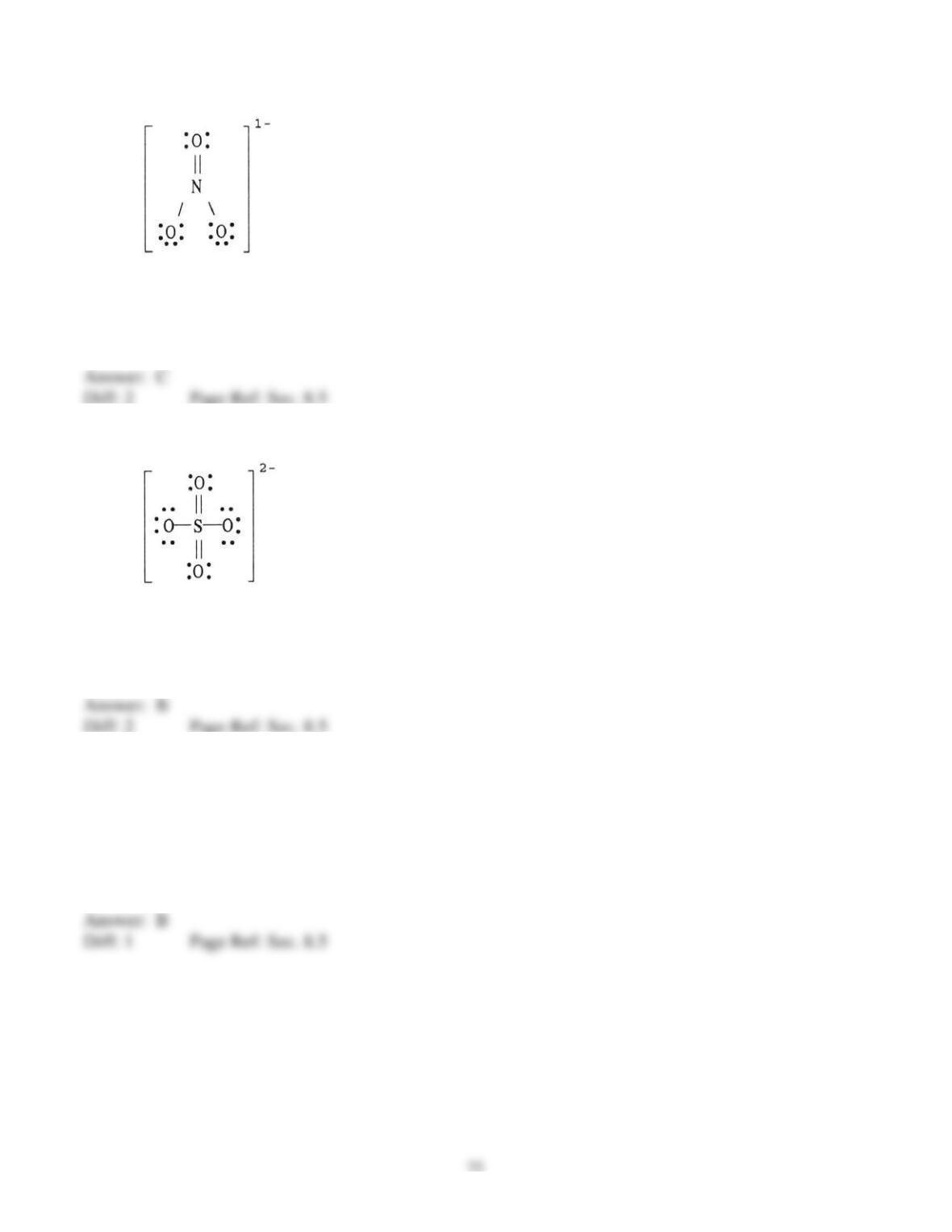
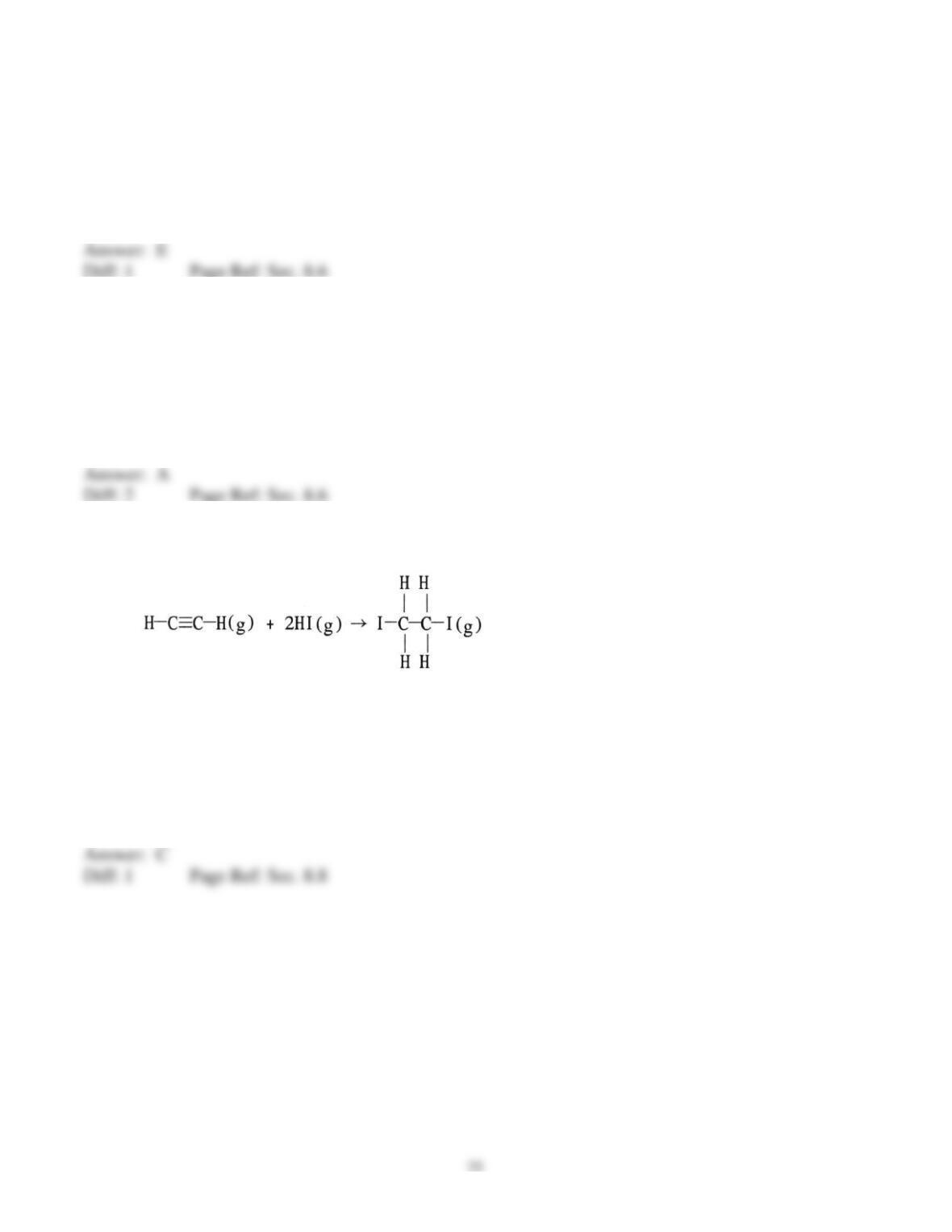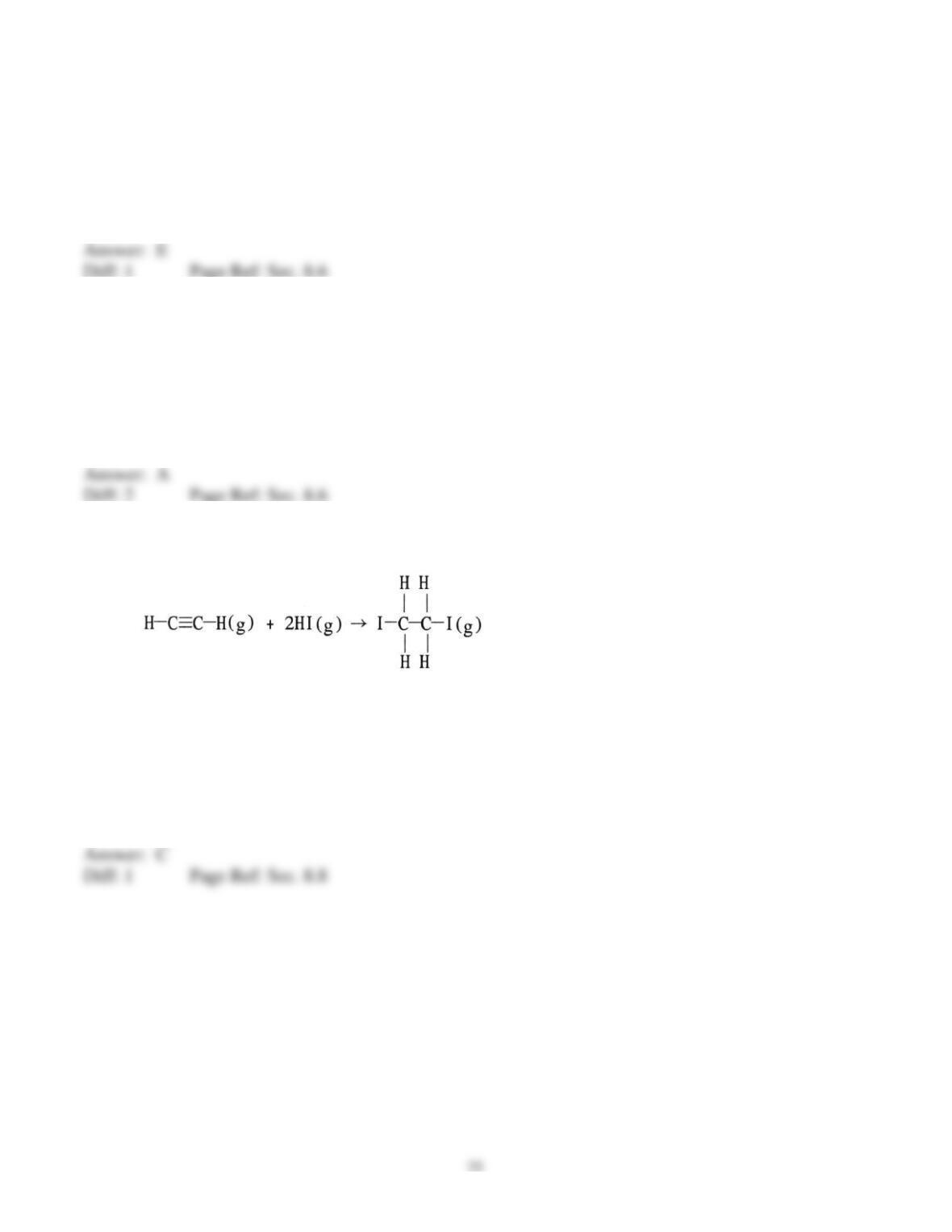6) Draw the Lewis structure of ICl2+.
7) Alternative but equivalent Lewis structures are called __________.
8) Benzene is an __________ compound with __________ equivalent Lewis structures.
9) In a reaction, if the bonds in the reactants are stronger than the bonds in the product, the reaction is
__________.
10) In compounds of __________ and __________, the octet rule is violated due to the presence of
fewer than eight valence electrons.
11) Polyatomic ions with an odd number of electrons will __________ the octet rule.
12) The strength of a covalent bond is measured by its __________.
13) To produce maximum heat, an explosive compound should have __________ chemical bonds and
decompose to molecule with __________ bonds.
14) Calculate the bond energy of C F given that the heat of atomization of CHFClBr is 1502 kJ/mol,
and that the bond energies of C H, C Br, and C Cl are 413, 276, and 328 kJ/mol, respectively.