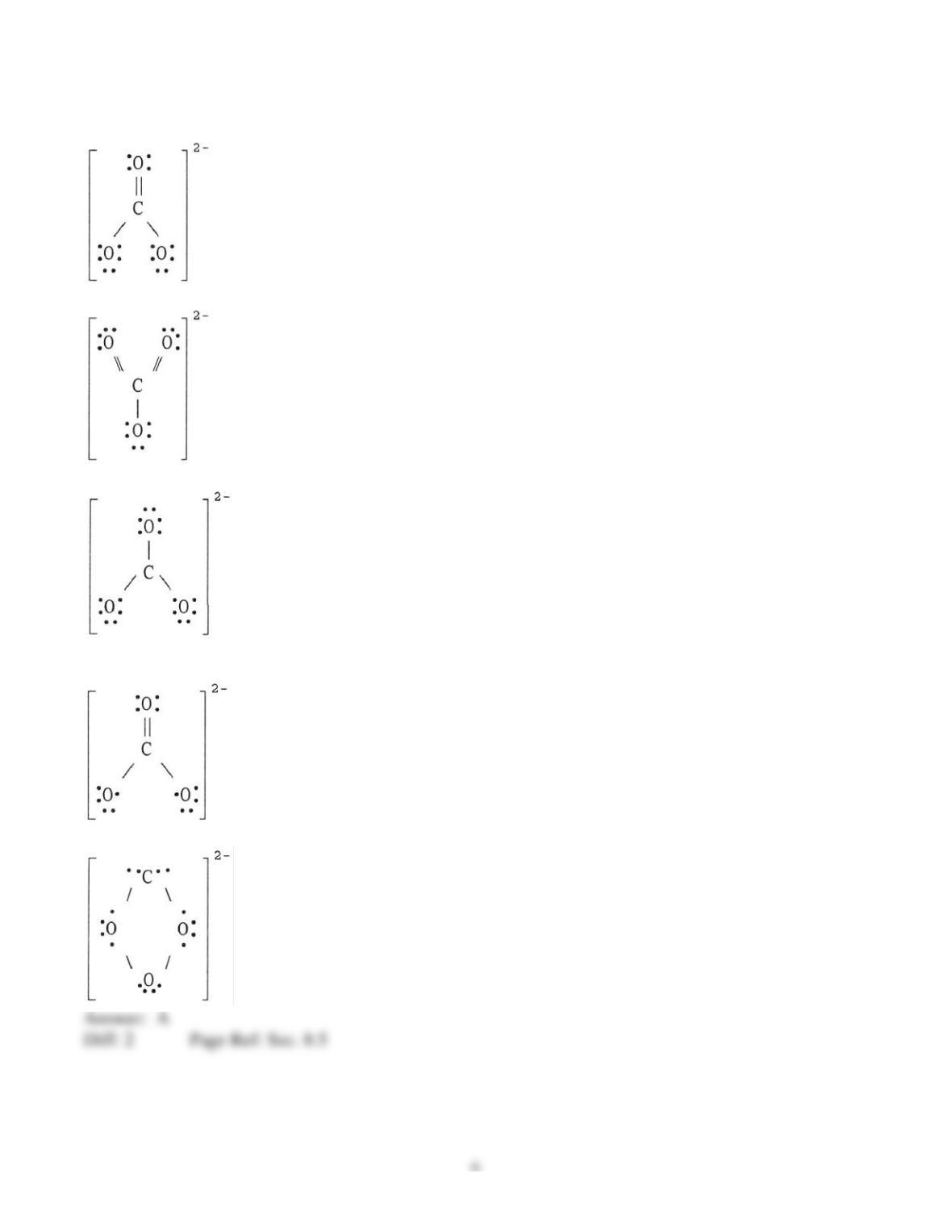
5) Lattice energy is __________.
A) the energy required to convert a mole of ionic solid into its constituent ions in the gas phase
B) the energy given off when gaseous ions combine to form one mole of an ionic solid
C) the energy required to produce one mole of an ionic compound from its constituent elements in their
standard states
D) the sum of ionization energies of the components in an ionic solid
E) the sum of electron affinities of the components in an ionic solid
6) In ionic bond formation, the lattice energy of ions ________ as the magnitude of the ion charges
_______ and the radii __________.
A) increases, decrease, increase
B) increases, increase, increase
C) decreases, increase, increase
D) increases, increase, decrease
E) increases, decrease, decrease
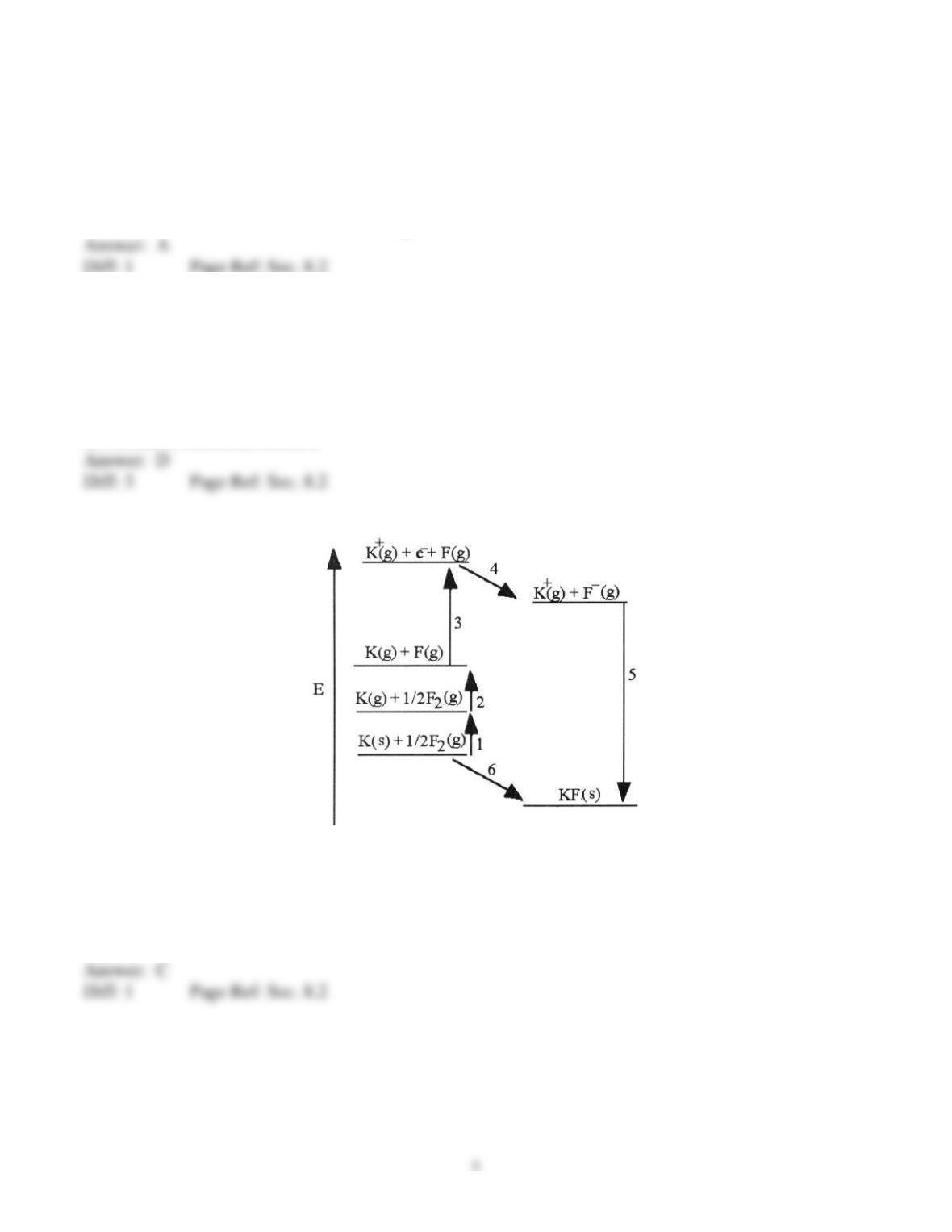
The diagram below is the Born-Huber cycle for the formation of crystalline potassium fluoride.
7) Which energy change corresponds to the electron affinity of fluorine?
A) 2
B) 5
C) 4
D) 1
E) 6