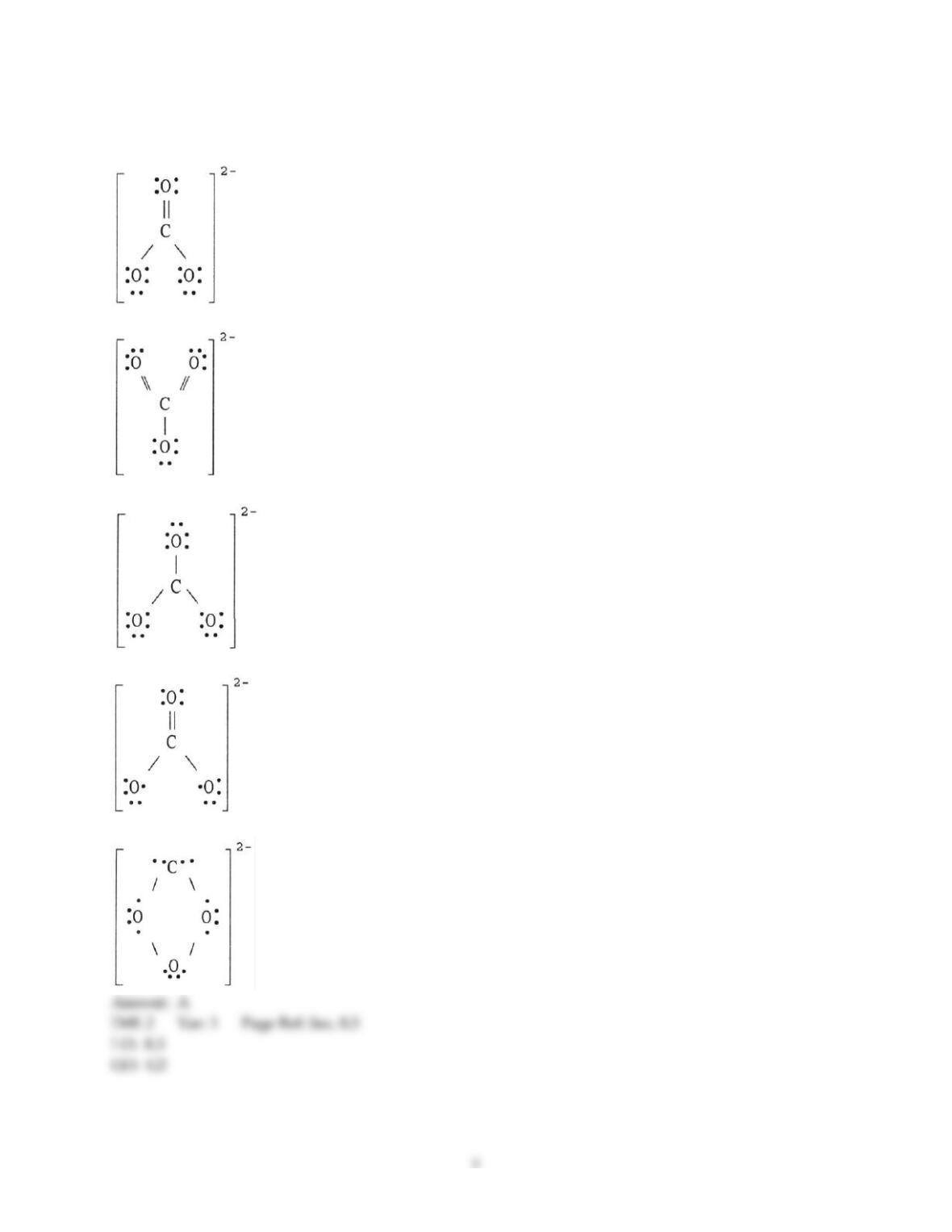
15) In the nitrite ion (NO2-), ________.
A) both bonds are single bonds
B) both bonds are double bonds
C) one bond is a double bond and the other is a single bond
D) both bonds are the same
E) there are 20 valence electrons
16) Resonance structures differ by ________.
A) number and placement of electrons
B) number of electrons only
C) placement of atoms only
D) number of atoms only
E) placement of electrons only
17) The oxidation number of iron in Fe2O3 is ________.
A) -2
B) +1
C) +3
D) +2
E) -3
18) To convert from one resonance structure to another, ________.
A) only atoms can be moved
B) electrons and atoms can both be moved
C) only electrons can be moved
D) neither electrons nor atoms can be moved
E) electrons must be added