109. Which of the following statements is true?
The first ionization potential of H is greater than that of He.
The ionic radius of Fe+ is larger than that of Fe3+.
The ionization energy of S2– is greater than that of Cl–.
The atomic radius of Li is larger than that of Cs.
110. Which of the following statements is false?
A sodium atom has a smaller radius than a potassium atom.
A neon atom has a smaller radius than an oxygen atom.
A fluorine atom has a smaller first ionization energy than an oxygen atom.
A cesium atom has a smaller first ionization energy than a lithium atom.
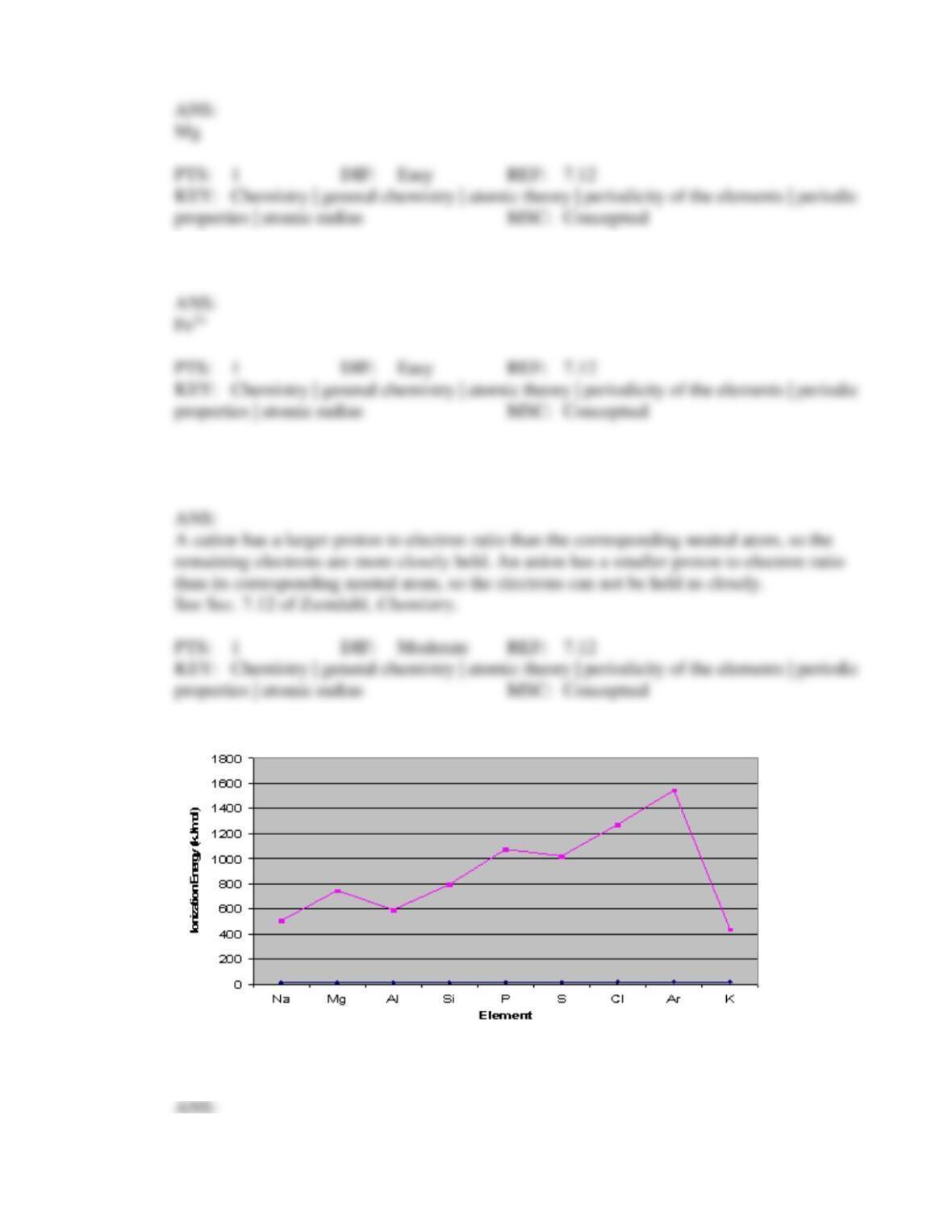
111. The statement that the first ionization energy for an oxygen atom is lower than the first
ionization energy for a nitrogen atom is
consistent with the general trend relating changes in ionization energy across a
period from left to right, because it is easier to take an electron from an oxygen
atom than from a nitrogen atom
consistent with the general trend relating changes in ionization energy across a
period from left to right, because it is harder to take an electron from an oxygen
atom than from a nitrogen atom
inconsistent with the general trend relating changes in ionization energy across a
period from left to right, due to the fact that the oxygen atom has two
doubly-occupied 2p orbitals and nitrogen has only one
inconsistent with the general trend relating changes in ionization energy across a
period from left to right, due to the fact that oxygen has one doubly-occupied 2p
orbital and nitrogen does not
112. Sodium losing an electron is an ________ process and fluorine losing an electron is an
_______ process.