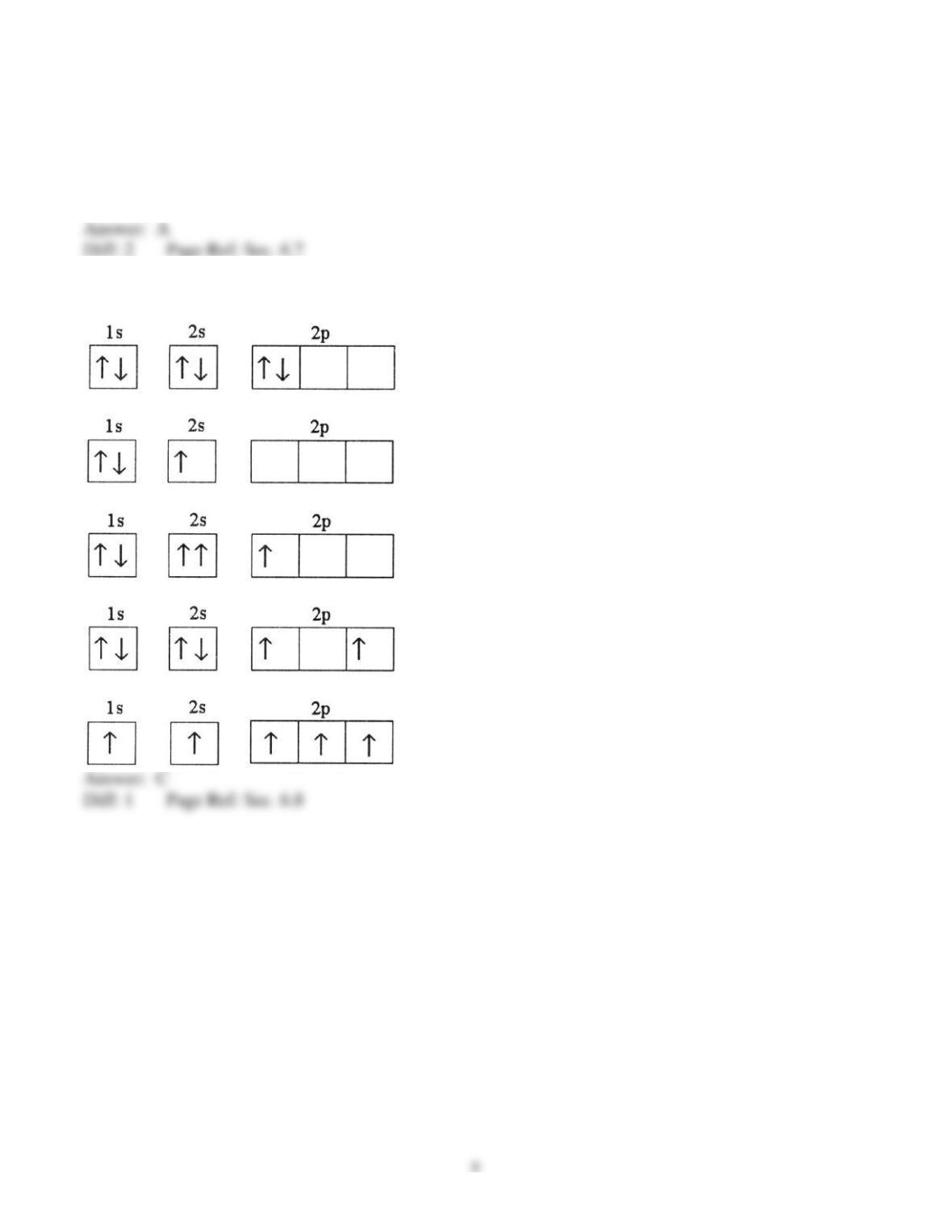
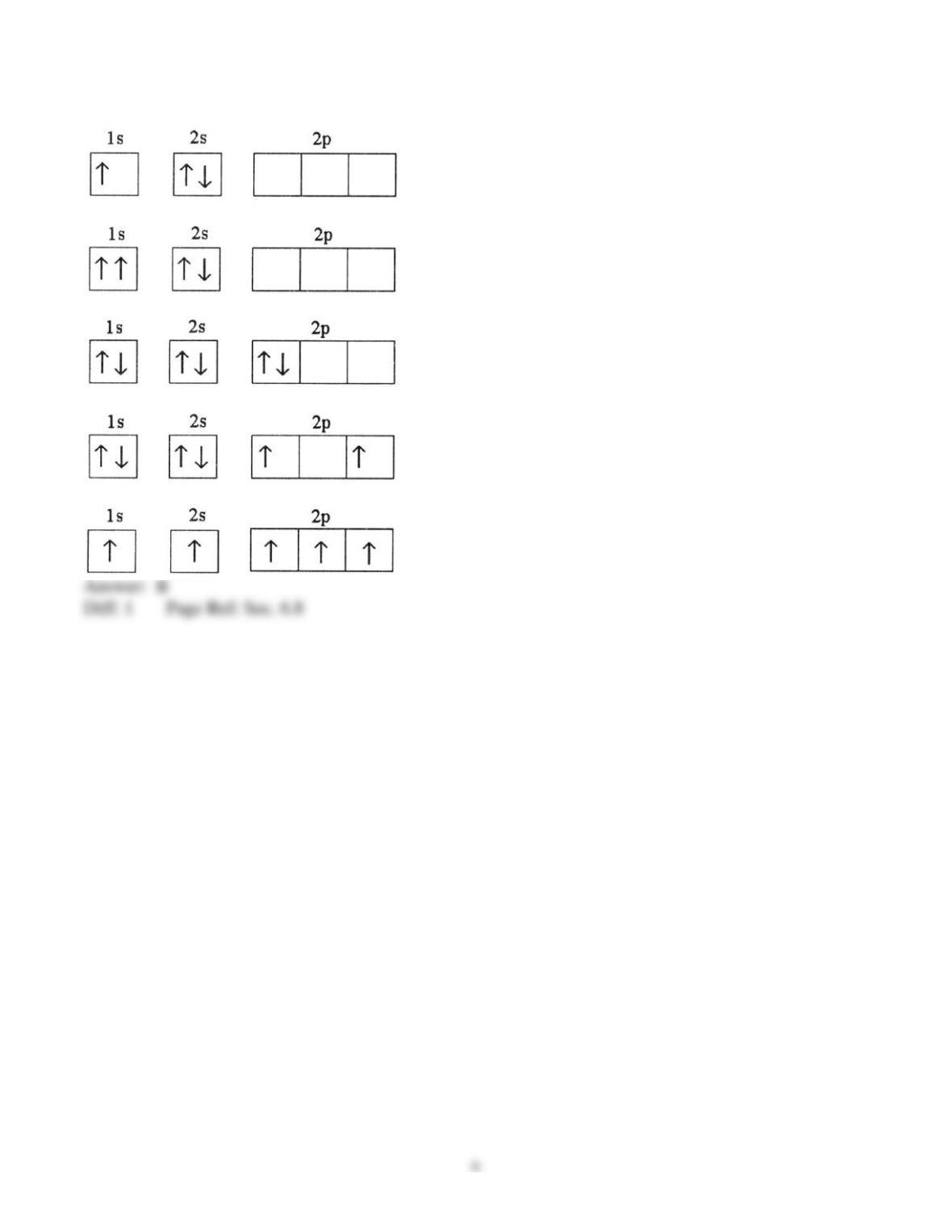
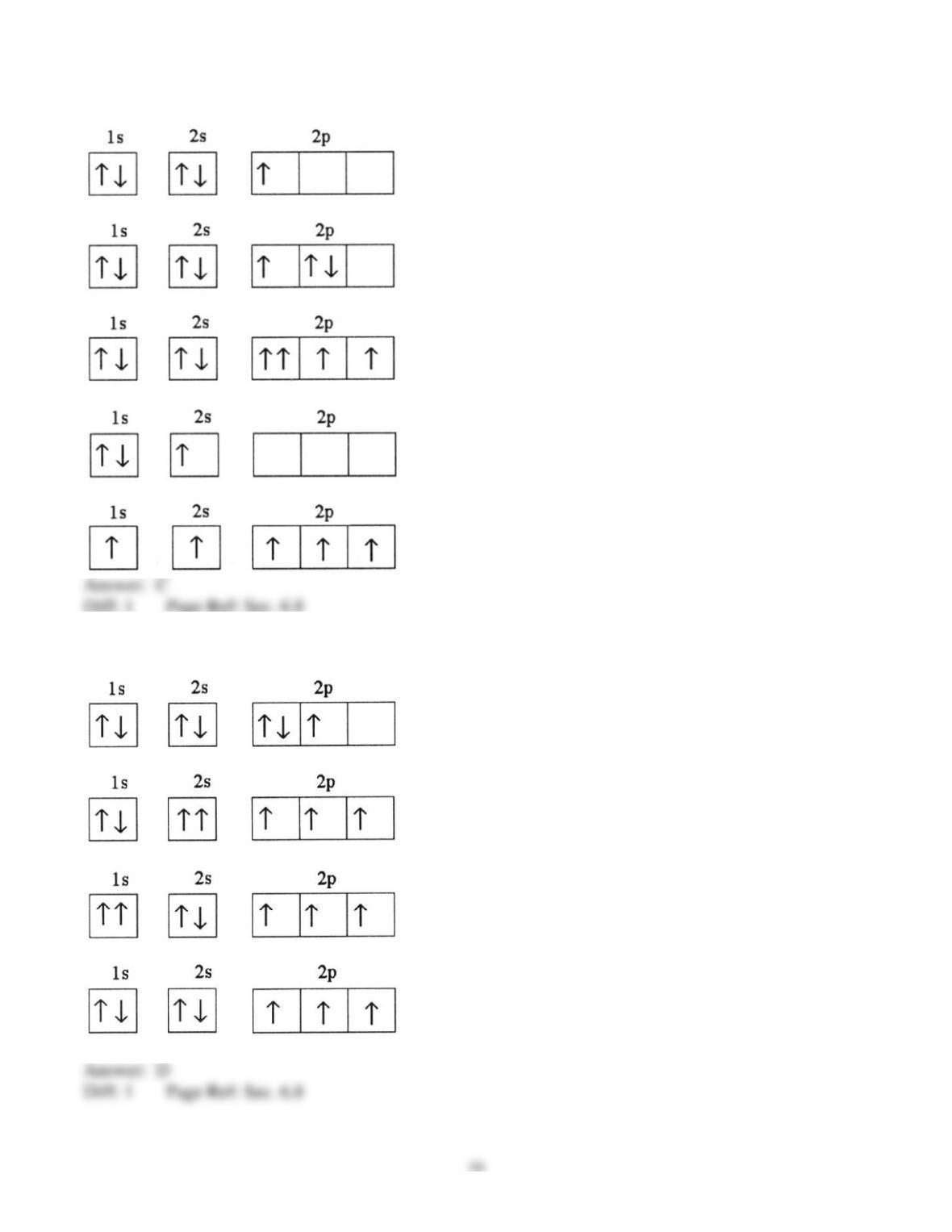
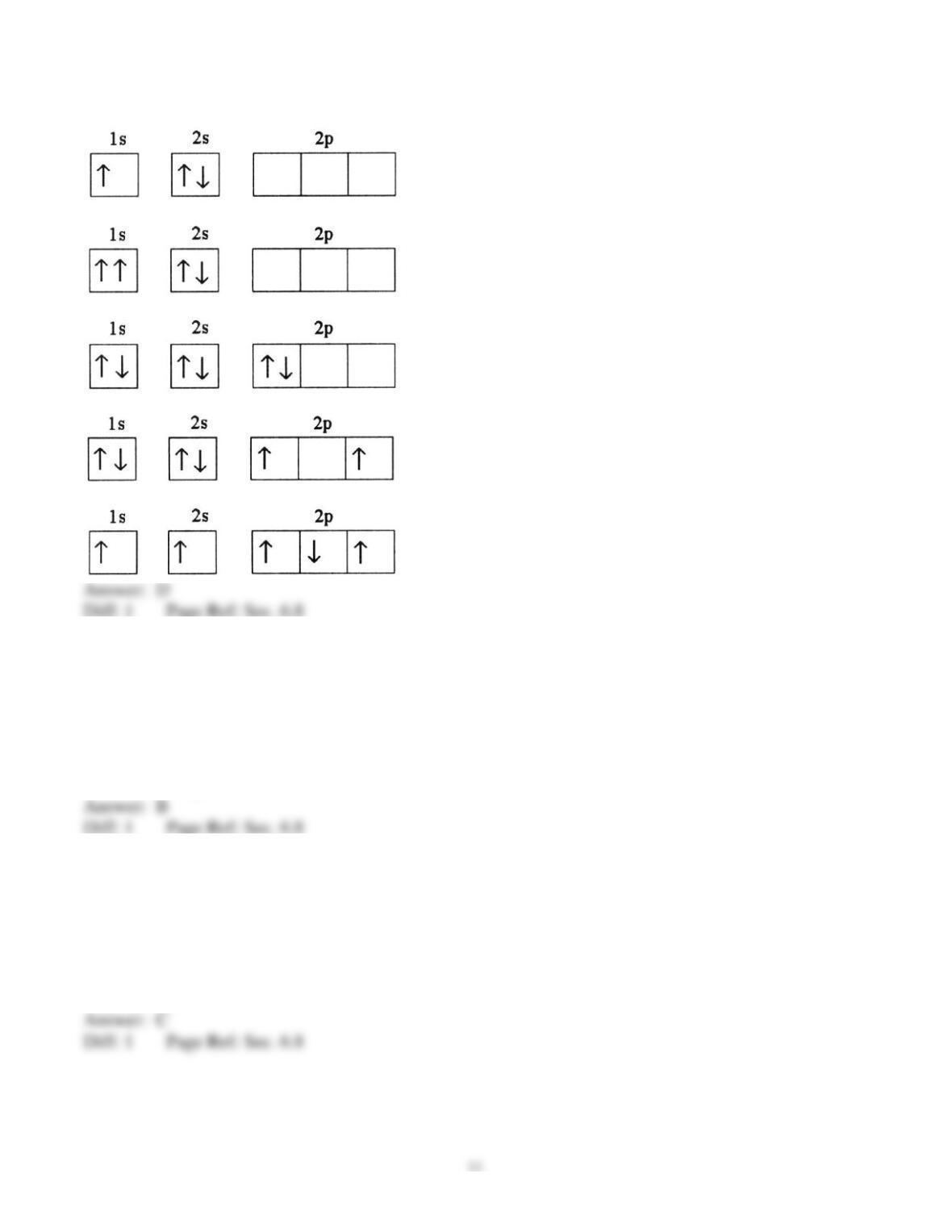
25) In a px orbital, the subscript x denotes the __________ of the electron.
A) energy
B) spin of the electrons
C) probability of the shell
D) size of the orbital
E) axis along which the orbital is aligned
26) The __________ orbital is degenerate with 5py in a many-electron atom.
A) 5s
B) 5px
C) 4py
D) 5dxy
E) 5d2
27) At maximum, an f-subshell can hold __________ electrons, a d-subshell can hold __________
electrons, and a p-subshell can hold __________ electrons.
A) 14, 10, 6
B) 2, 8, 18
C) 14, 8, 2
D) 2, 12, 21
E) 2, 6, 10
28) If an electron has a principal quantum number (n) of 3 and an angular momentum quantum number
(l) of 2, the subshell designation is __________.
A) 3p
B) 3d
C) 4s
D) 4p
E) 4d
29) Which one of the following represents an acceptable set of quantum numbers for an electron in an
atom? (arranged as n, l, ml, and ms)
A) 2, 2, -1, -1/2
B) 1, 0, 0, 1/2
C) 3, 3, 3, 1/2
D) 5, 4,- 5, 1/2
E) 3, 3, 3, -1/2