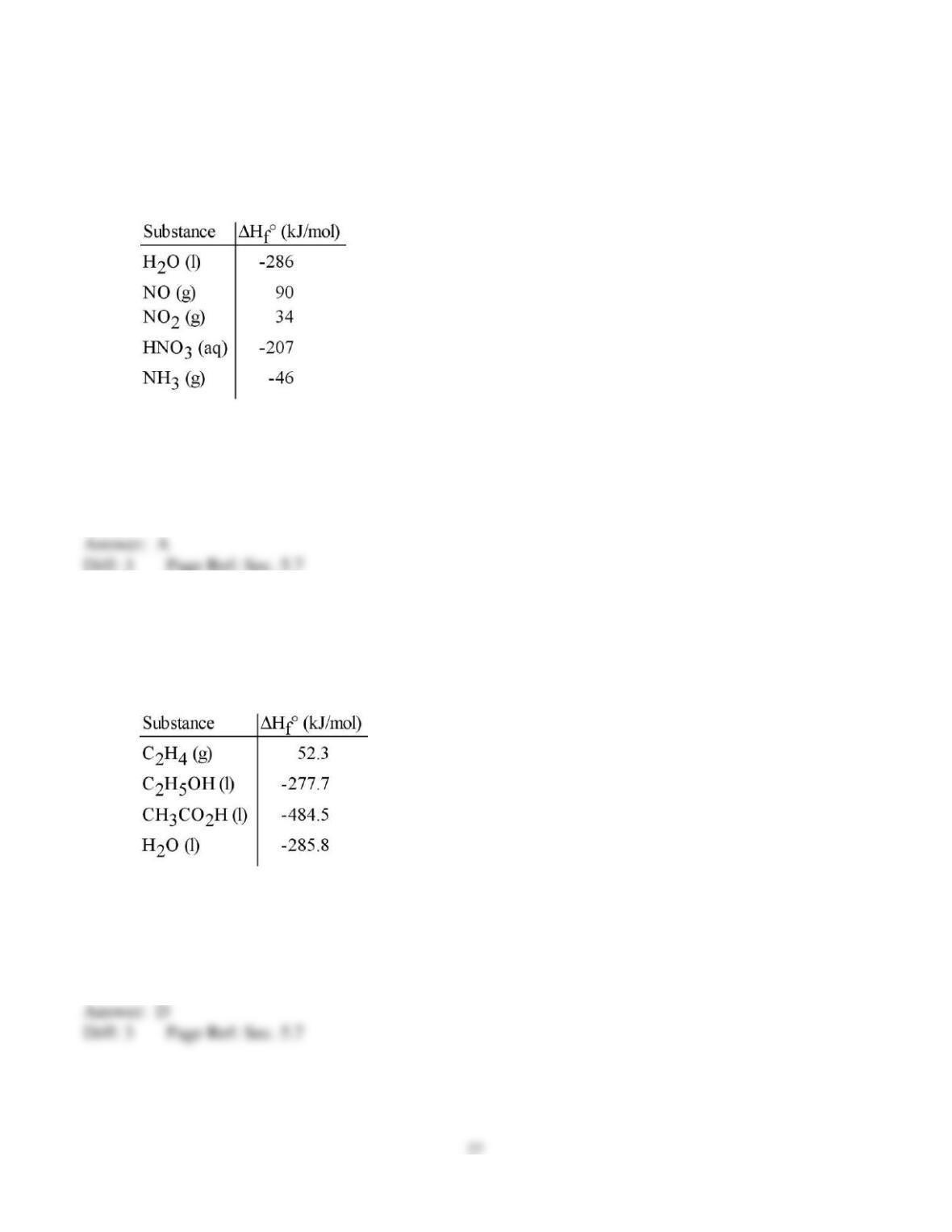
40) Given the following reactions
CaCO3 (s) → CaO (s) + CO2 (g) ΔH = 178.1 kJ
C (s, graphite) + O2 (g) → CO2 (g) ΔH = -393.5 kJ
the enthalpy of the reaction
CaCO3 (s) → CaO (s) + C (s, graphite) + O2 (g)
is __________ kJ.
A) 215.4
B) 571.6
C) -215.4
D) -571.6
E) 7.01 × 104
41) Given the following reactions
H2O (l) → H2O (g) ΔH = 44.01 kJ
2 H2 (g) + O2 (g) → 2 H2O (g) ΔH = -483.64 kJ
the enthalpy for the decomposition of liquid water into gaseous hydrogen and oxygen
2 H2O (l) → 2 H2 (g) + O2 (g)
is __________ kJ.
A) -395.62
B) -527.65
C) 439.63
D) 571.66
E) 527.65
42) Given the following reactions
N2 (g) + O2 (g) → 2NO (g) ΔH = +180.7 kJ
2NO( g) + O2 (g) → 2NO2 (g) ΔH = -113.1 kJ
the enthalpy for the decomposition of nitrogen dioxide into molecular nitrogen and oxygen
2NO2 (g) → N2 (g) + 2 O2 (g)
is __________ kJ.
A) 67.6
B) -67.6
C) 293.8
D) -293.8
E) 45.5