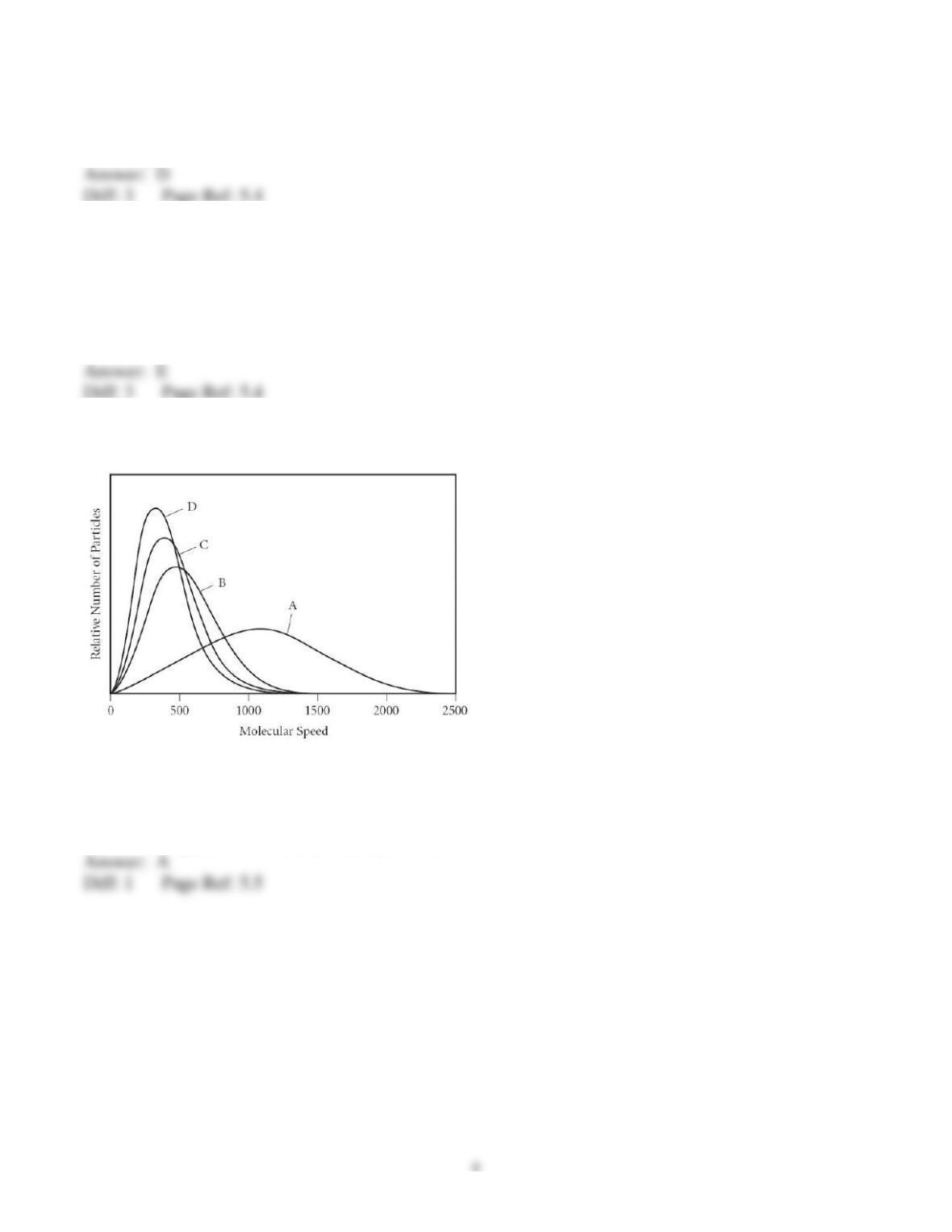
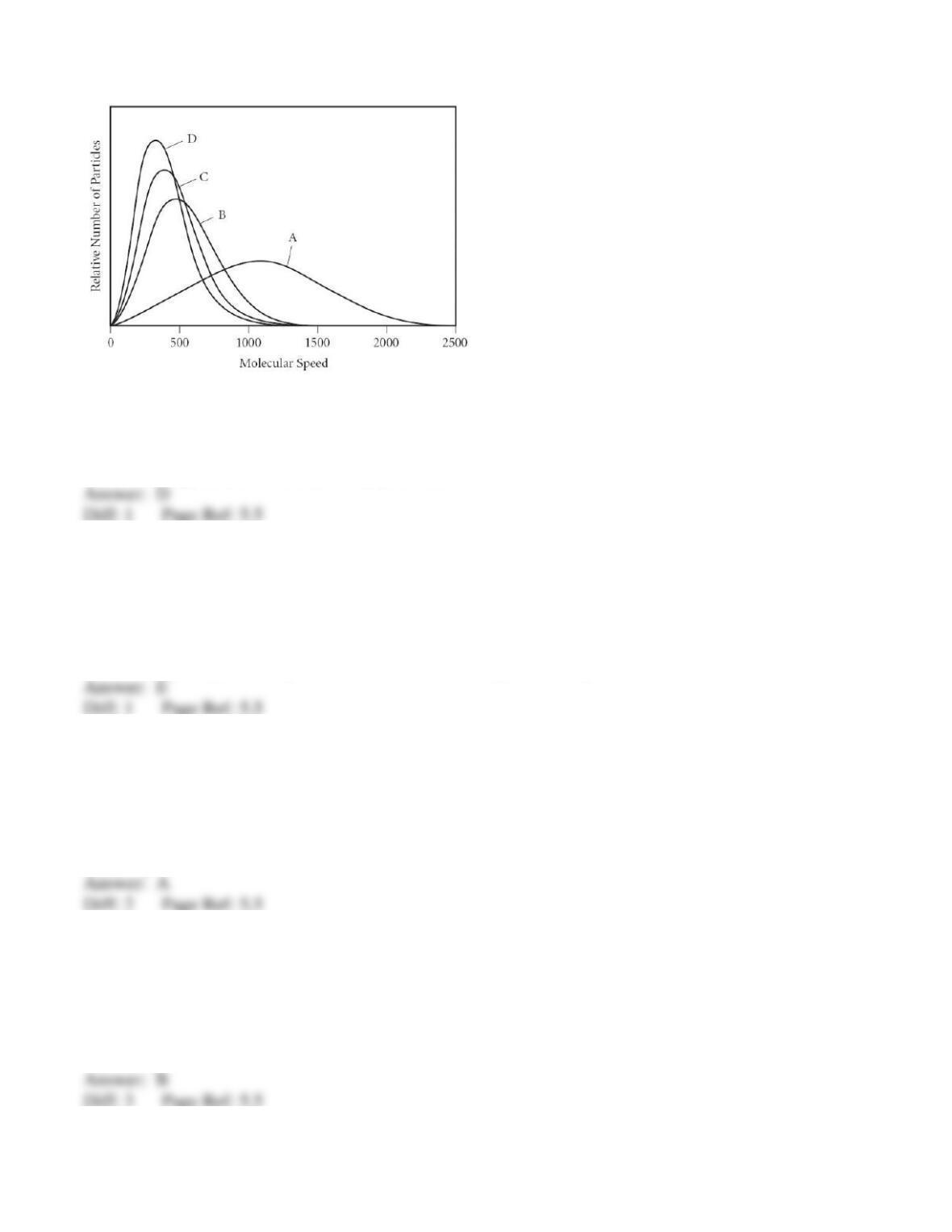
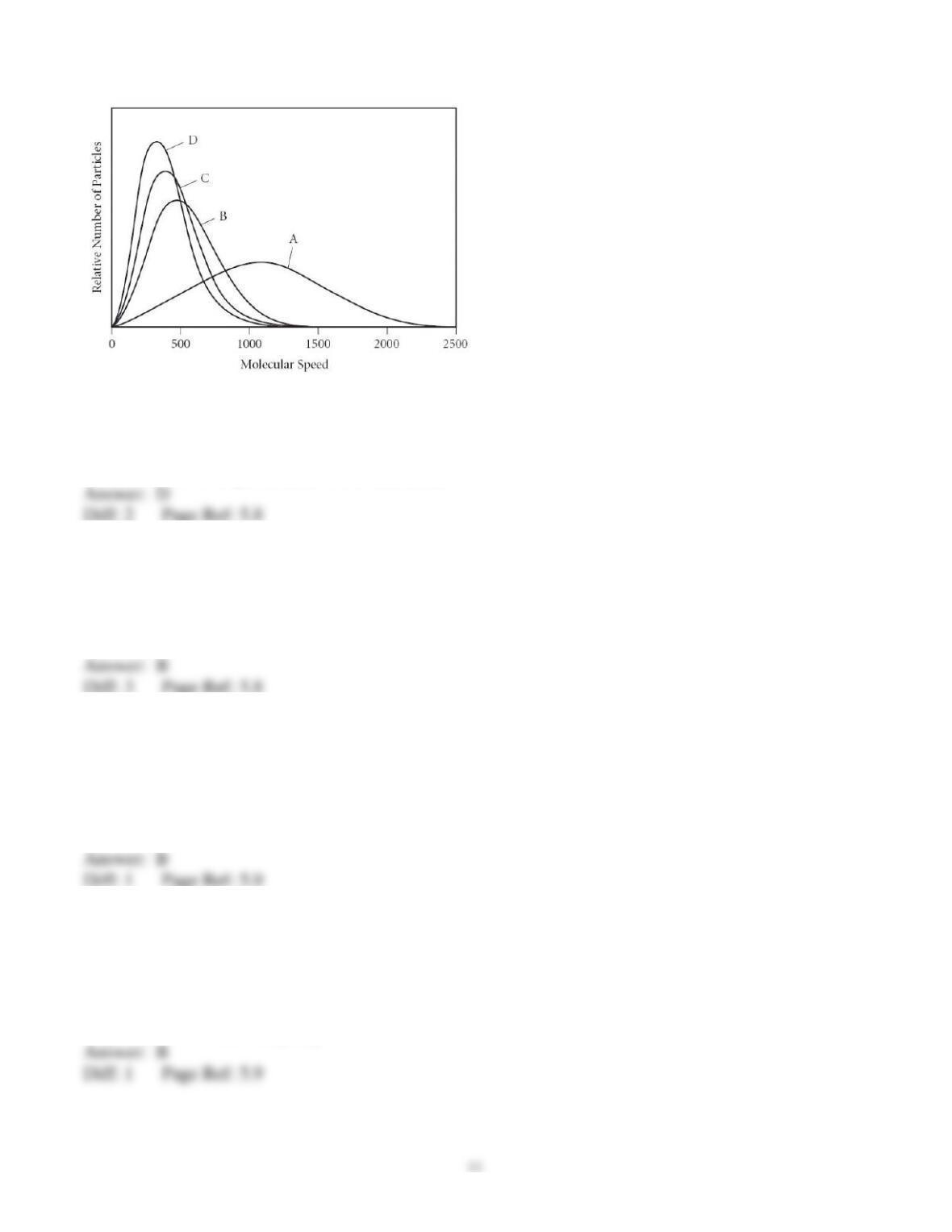
47) The rate of effusion of oxygen to an unknown gas is 0.935. What is the other gas?
A) Ne
B) Ar
C) F2
D) N2
48) Which of the following statements is TRUE?
A) Particles of different masses have the same average speed at a given temperature.
B) The larger a molecule, the faster it will effuse.
C) At very high pressures, a gas will occupy a larger volume than predicted by the ideal gas law.
D) For a given gas, the lower the temperature, the faster it will effuse.
E) None of the above statements are true.
49) Which of the following statements is TRUE?
A) At a given temperature, lighter gas particles travel more slowly than heavier gas particles.
B) The smaller a gas particle, the slower it will effuse
C) The higher the temperature, the lower the average kinetic energy of the sample.
D) At low temperatures, intermolecular forces become important and the pressure of a gas will be lower
than predicted by the ideal gas law.
E) None of the above statements are true.
50) Which of the following compounds will behave LEAST like an ideal gas at low temperatures?
A) He
B) SO2
C) H2
D) N2
E) F2
51) This equation is used to calculate the properties of a gas under nonideal conditions.
A) Charles's Law
B) Avogadro's Law
C) Boyle's Law
D) van der Waals equation
E) Dalton's Law