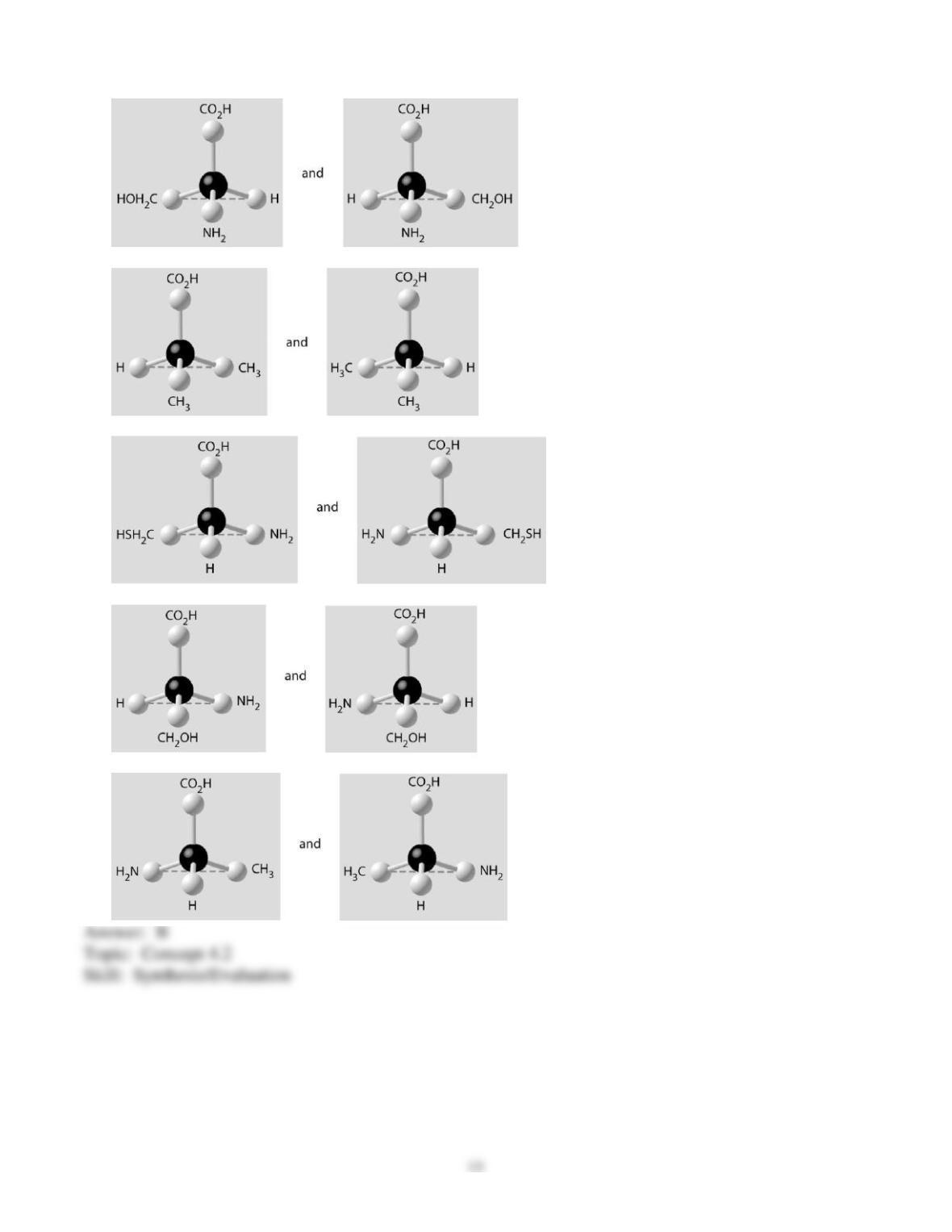
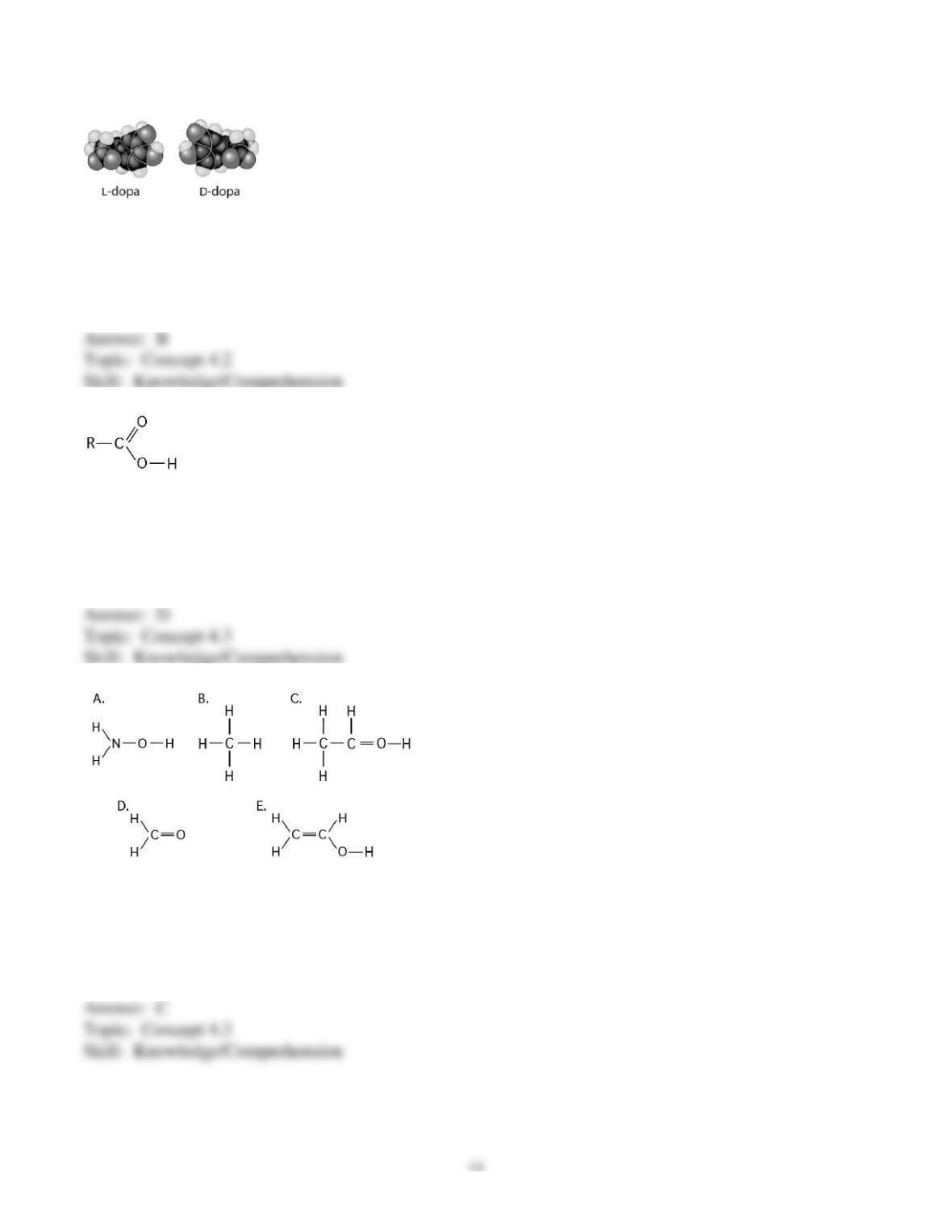
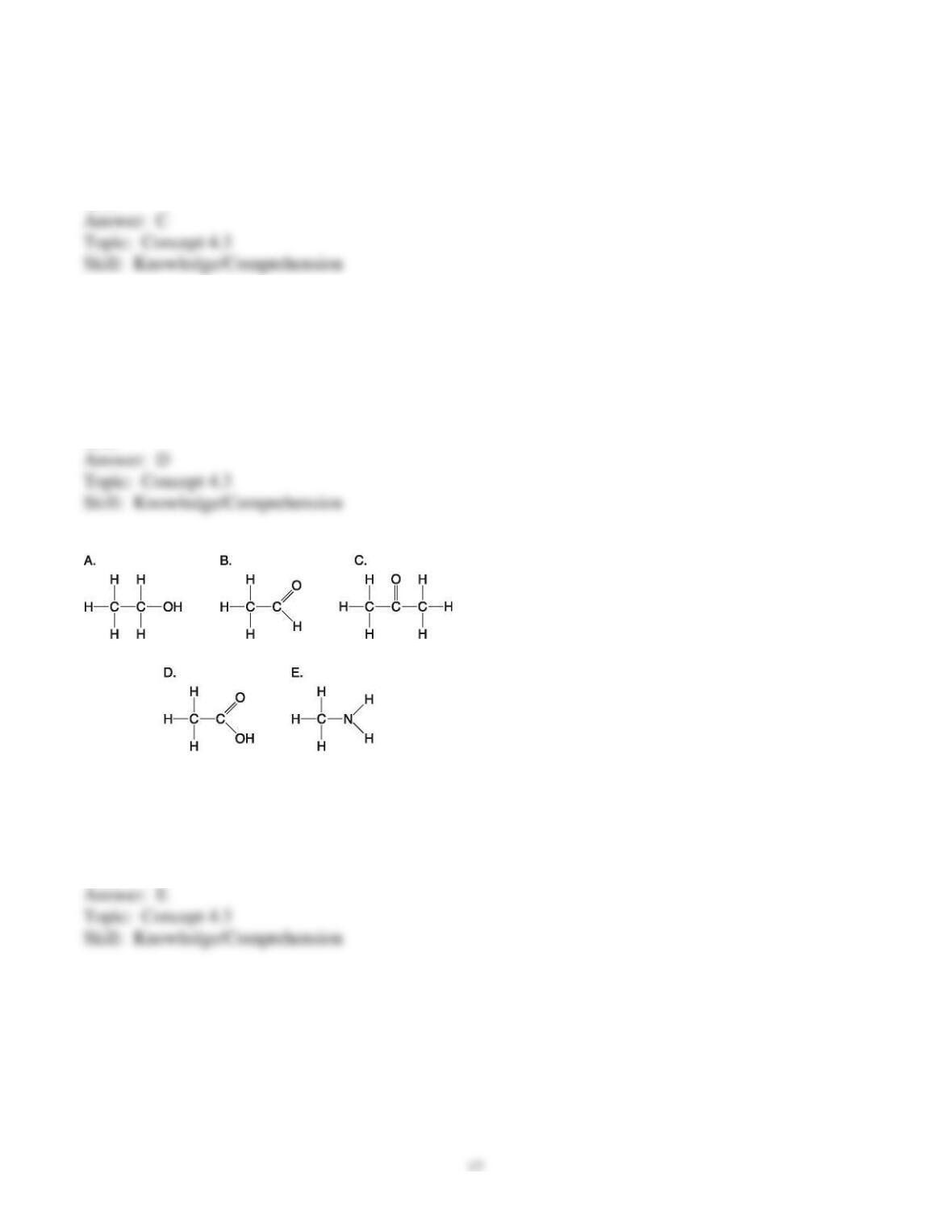
19
therefore an organic acid?
A) A
B) B
C) C
D) D
E) E
58) Which molecule shown above can form a dimer linked by a covalent bond?
A) A
B) B
C) C
D) D
E) E
59) Which molecules shown above will form hydrogen bonds with water?
A) Only D will form hydrogen bonds with water.
B) All of these molecules will form hydrogen bonds with water.
C) None of these molecules will form hydrogen bonds with water.
D) All of these molecules except B will form hydrogen bonds with water.
E) Only C, D, and E will form hydrogen bonds with water.
60) Which molecule shown above contains an amino functional group, but is not an amino acid?
A) A
B) B
C) C
D) D
E) E
61) Which molecule shown above is a thiol?