Campbell's Biology, 9e (Reece et al.)
Chapter 4 Carbon and the Molecular Diversity of Life
This chapter focuses on the chemistry of carbon and organic compounds. Students should be able to
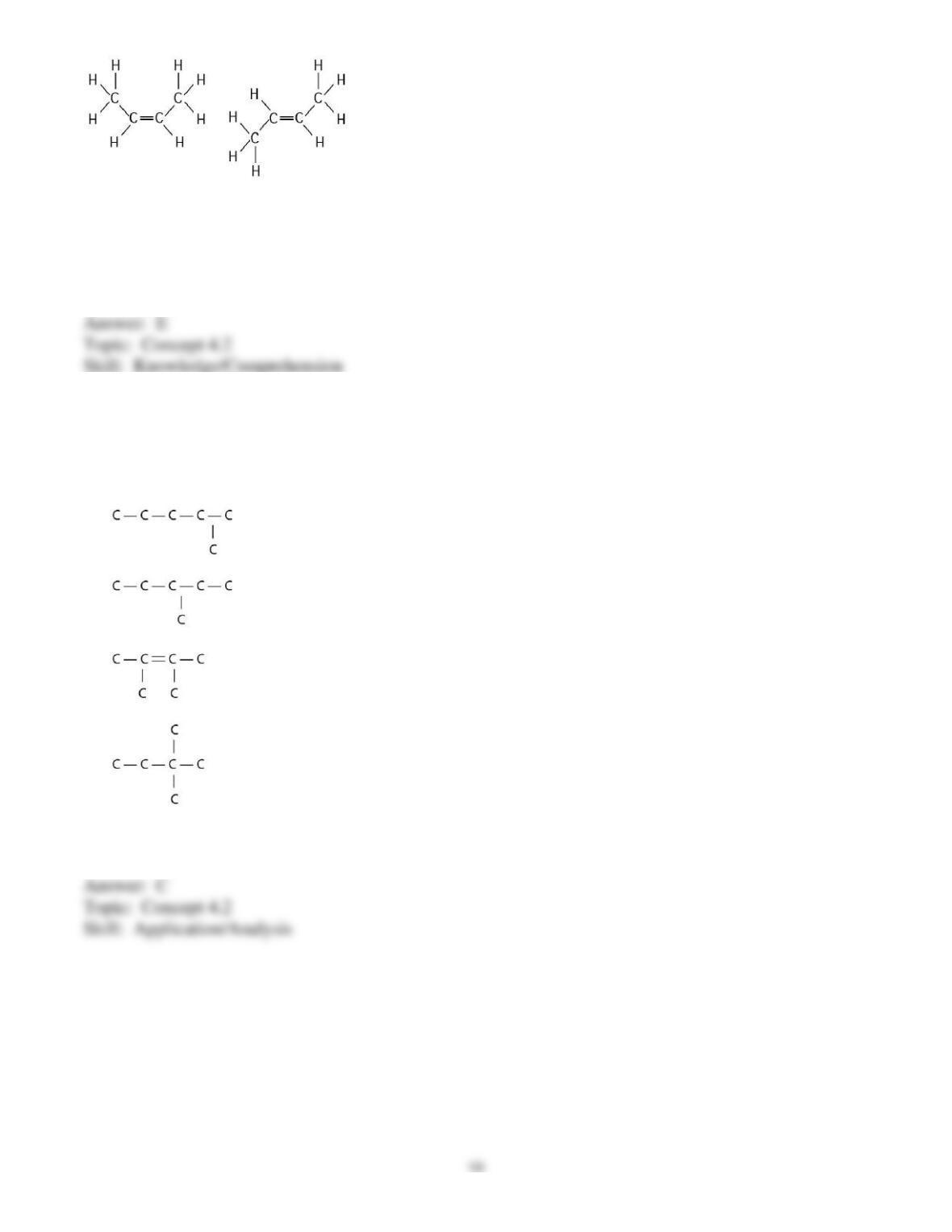
identify the nature of the bonds between carbon and other elements (nonpolar versus polar), the different
types of weak bonds and interactions, the various types of isomers, the basic functional groups of
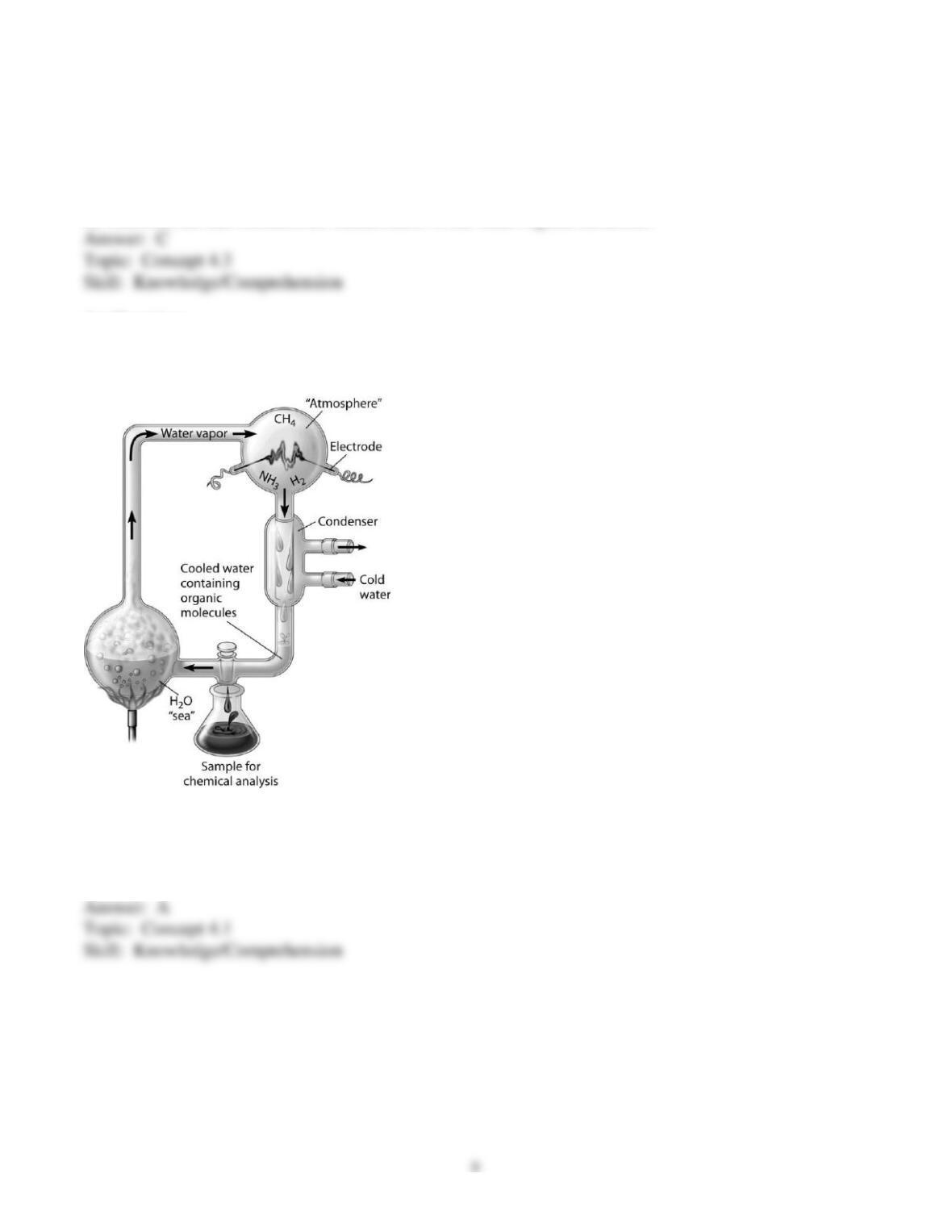
organic molecules, and their relative solubility in water. The abiotic formation of organic molecules
from inorganic molecules is important in the origin of life.
Multiple-Choice Questions
1) The element present in all organic molecules is
A) hydrogen.
B) oxygen.
C) carbon.
D) nitrogen.
E) phosphorus.
2) The complexity and variety of organic molecules is due to
A) the chemical versatility of carbon atoms.
B) the variety of rare elements in organic molecules.
C) the fact that they can be synthesized only in living organisms.
D) their interaction with water.
E) their tremendously large sizes.
3) The experimental approach taken in current biological investigations presumes that
A) simple organic compounds can be synthesized in the laboratory from inorganic precursors, but
complex organic compounds like carbohydrates and proteins can only be synthesized by living
organisms.
B) a life force ultimately controls the activities of living organisms and this life force cannot be studied
by physical or chemical methods.
C) although a life force, or vitalism, exists in living organisms, this life force cannot be studied by
physical or chemical methods.
D) living organisms are composed of the same elements present in nonliving things, plus a few special
trace elements found only in living organisms or their products.
E) living organisms can be understood in terms of the same physical and chemical laws that can be used
to explain all natural phenomena.