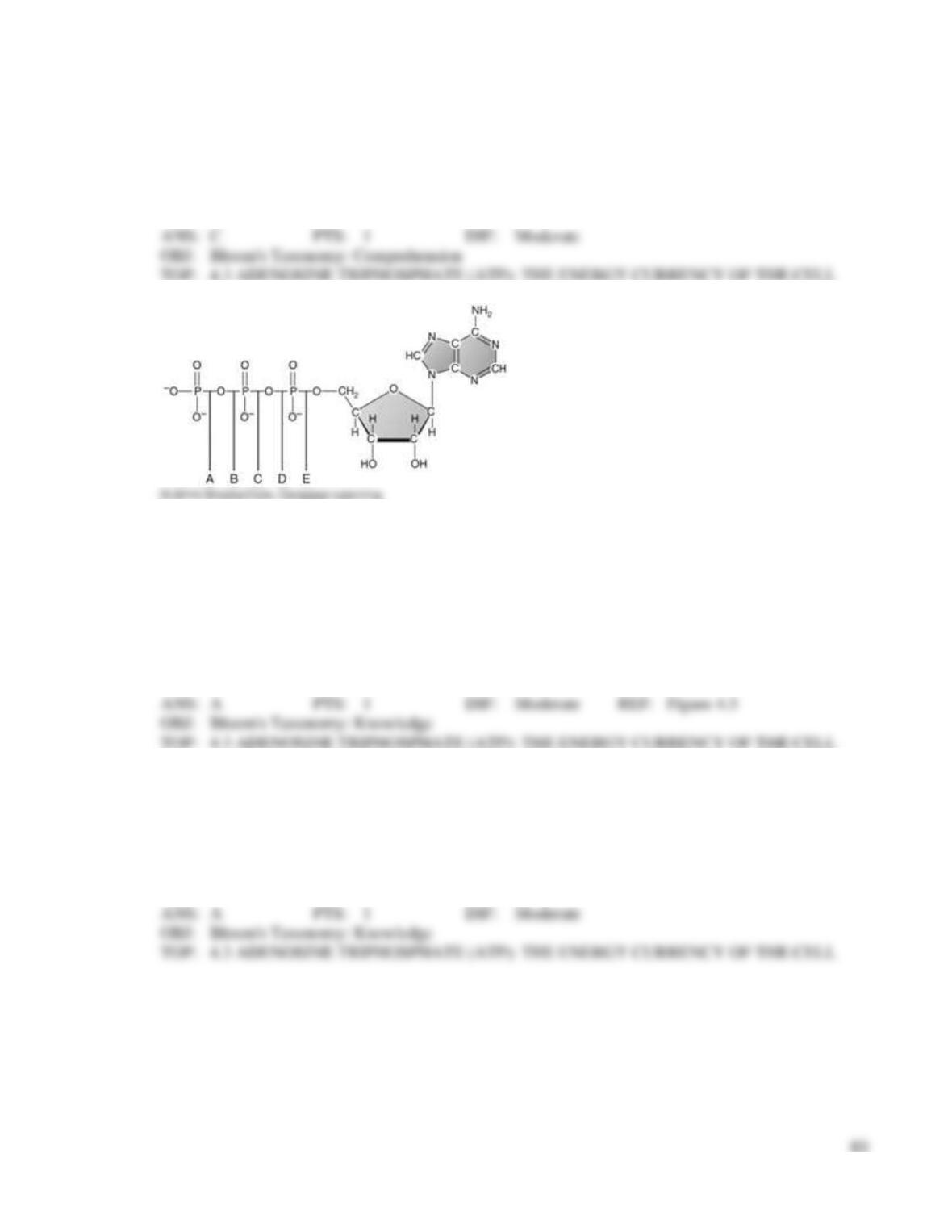
43. You do an experiment in the laboratory and add increasing amounts of substrate to a solution
containing an enzyme and a pH buffer. You incubate the container at the optimal temperature for your
enzyme. Each time you add more substrate, you measure the rate of the reaction. If you graph the
results where the x-axis shows the substrate concentration and the y-axis shows the resulting reaction
rate, what will you find over time?
The rate of the reaction will proceed with a slope of 1 and continue in a linear fashion
indefinitely or until you run out of reactants.
The rate of the reaction will increase rapidly, taper off, and plateau.
The rate of the reaction will increase slowly, plateau, and then drop sharply back to zero.
The resulting graph will be a perfect bell curve.
There is no way to predict what the graph will look like without more information.
44. In competitive inhibition
the products of the reaction block the active site of the enzyme.
the products of the reaction bind to a site other than the active site of the enzyme and
block enzyme activity indirectly.
the substrate and cofactors compete for the active site.
the inhibitor binds to and directly blocks the active site of the enzyme.
the inhibitor binds to an enzyme at a site other than its active site.
45. The cofactors required for enzyme activity often are
large, complex organic molecules.
46. In noncompetitive feedback inhibition
the products of the reaction block the active site of the enzyme.
the products of the reaction at the end of the pathway bind to a site other than the active
site of an enzyme at or near the beginning of the pathway and block enzyme activity
indirectly.
the substrate and cofactors compete for the active site.
the inhibitor binds to and directly blocks the active site of the enzyme.
enzyme activity is controlled by the reversible binding of a regulatory molecule.