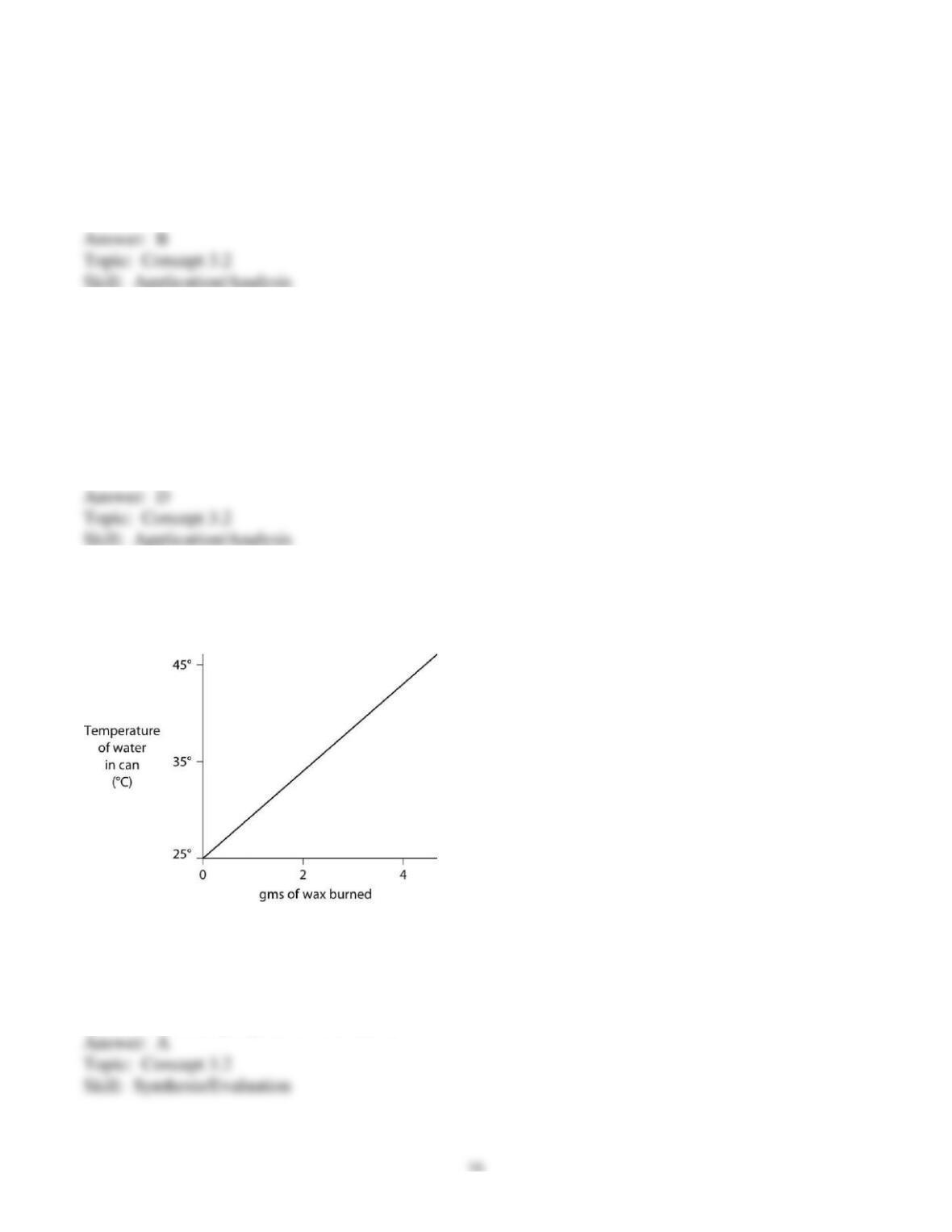
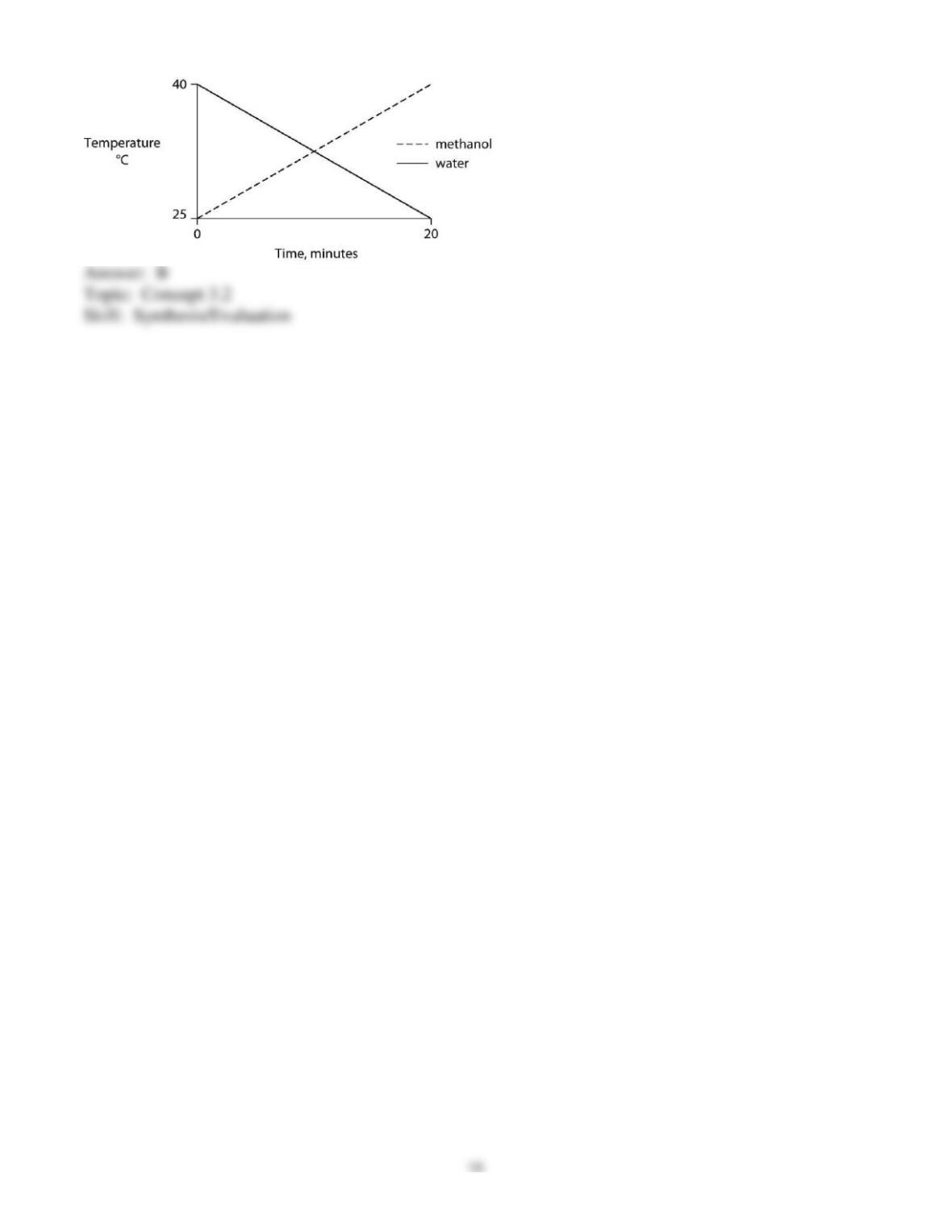
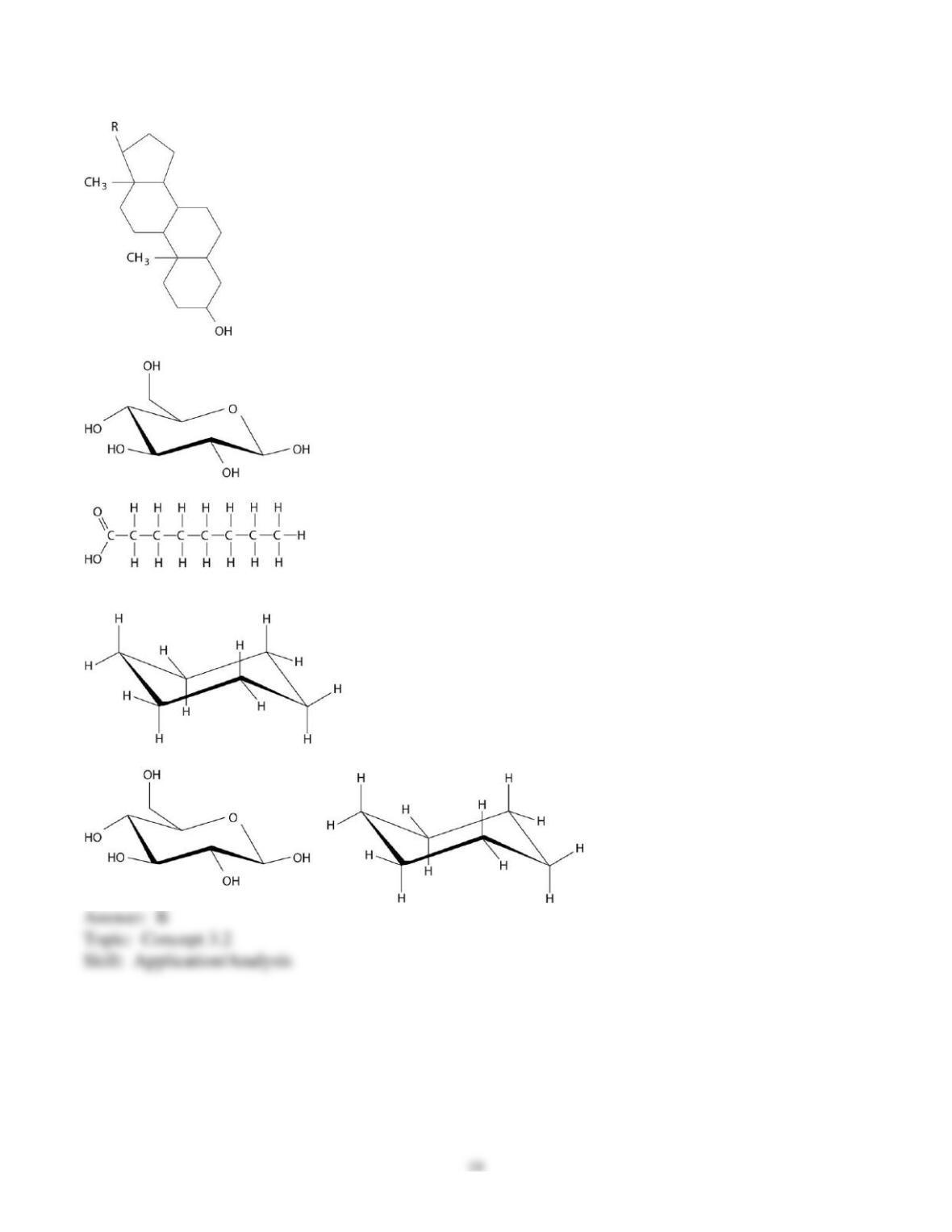
46) Carbon dioxide (CO2) is readily soluble in water, according to the equation CO2 + H2O ↔ H2CO3.
Carbonic acid (H2CO3) is a weak acid. Respiring cells release CO2 into the bloodstream. What will be
the effect on pH of blood as that blood first comes in contact with respiring cells?
A) Blood pH will decrease slightly.
B) Blood pH will increase slightly.
C) Blood pH will remain unchanged.
D) Blood pH will first increase, then decrease as CO2 combines with hemoglobin.
E) Blood pH will first decrease, then increase sharply as CO2 combines with hemoglobin.
47) A beaker contains 100 mL of NaOH solution at pH = 13. A technician carefully pours into the
beaker 10 mL of HCl at pH = 1. Which of the following statements correctly describes the results of this
mixing?
A) The concentration of Na+ ion rises.
B) The concentration of Cl- ion will be 0.1 M.
C) The concentration of undissociated H2O molecules remains unchanged.
D) The pH of the beaker's contents will be neutral.
E) The pH of the beaker's contents falls.
48) Equal volumes (5 mL) of vinegar from a freshly opened bottle are added to each of the following
solutions. After complete mixing, which of the mixtures will have the highest pH?
A) 100 mL of pure water
B) 100 mL of freshly brewed coffee
C) 100 mL of household cleanser containing 0.5 M ammonia
D) 100 mL of freshly squeezed orange juice
E) 100 mL of tomato juice
49) Increased atmospheric CO2 concentrations might have what effect on seawater?
A) Seawater will become more acidic, and bicarbonate concentrations will decrease.
B) Seawater will become more alkaline, and carbonate concentrations will decrease.
C) There will be no change in the pH of seawater, because carbonate will turn to bicarbonate.
D) Seawater will become more acidic, and carbonate concentrations will decrease.
E) Seawater will become more acidic, and carbonate concentrations will increase.