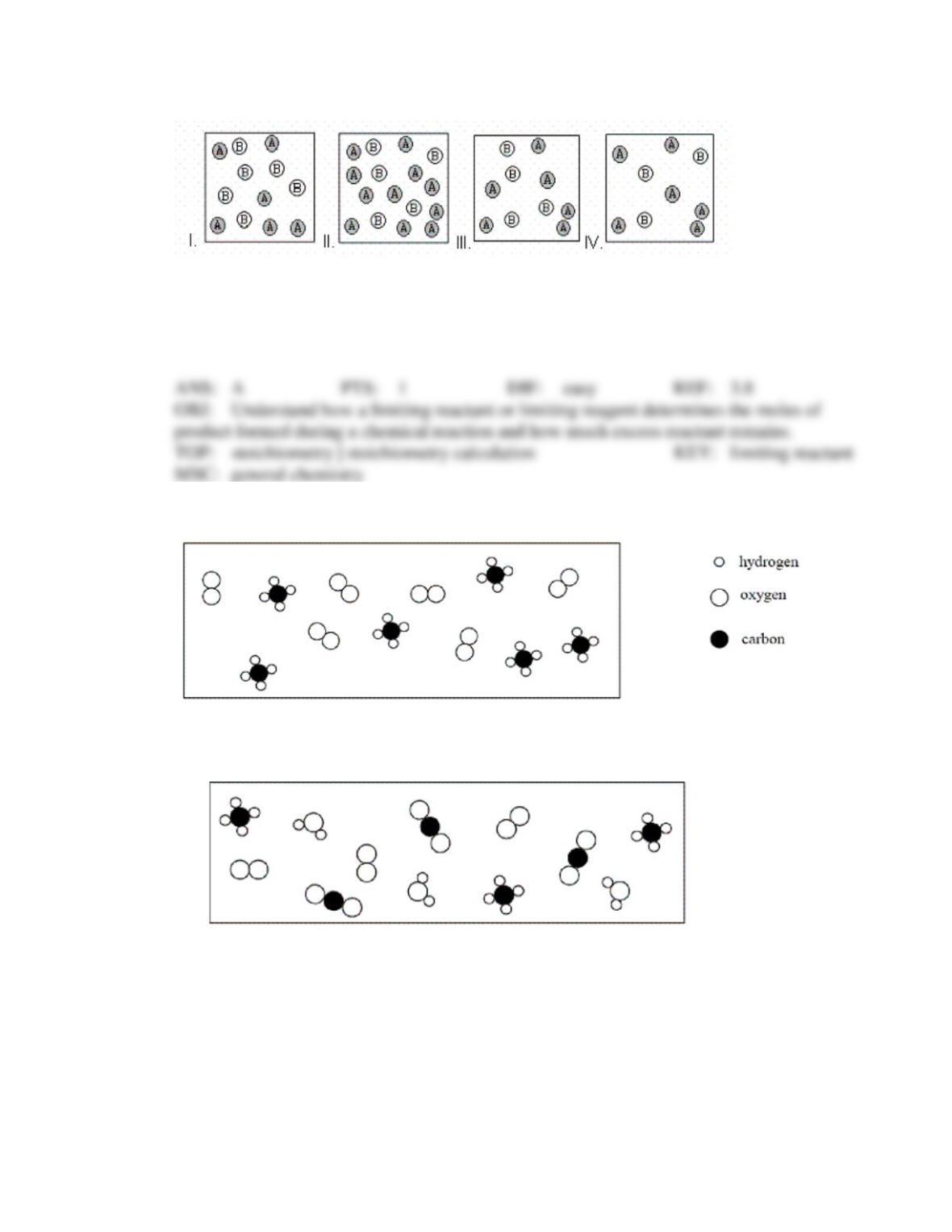
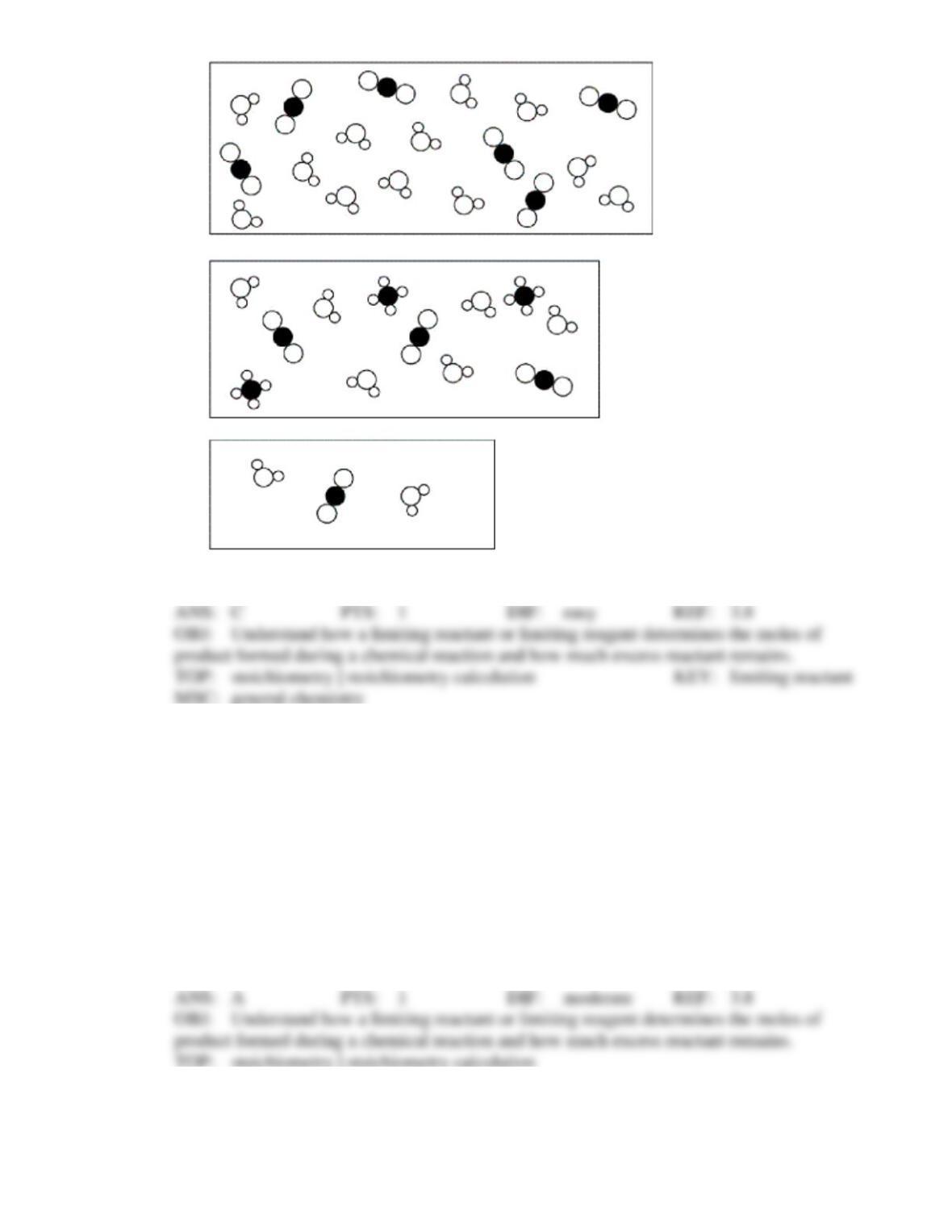
Test Bank General Chemistry, 10th edition 37
that has the lowest coefficient in the balanced equation.
that has the lowest molar mass.
that is left over after the reaction has gone to completion.
for which there is the lowest mass in grams.
130. The commercial production of phosphoric acid, H3PO4, can be represented by the equation
→ 3CaSiO3 + 5CO2 + 2H3PO4
The molar mass for each reactant is shown below the reactant, and the mass of each reactant
for this problem is given above. Which substance is the limiting reactant?
131. SO2 reacts with H2S as follows:
2H2S + SO2 → 3S + 2H2O
When 7.50 g of H2S reacts with 12.75 g of SO2, which statement applies?
6.38 g of sulfur is formed.
SO2 is the limiting reagent.
0.0216 mol of H2S remains.
10.6 g of sulfur is formed.
132. A 15-g sample of lithium is reacted with 15 g of fluorine to form lithium fluoride:
2Li + F2 → 2LiF. After the reaction is complete, what will be present?
0.789 mol of lithium fluoride only
2.16 mol of lithium fluoride only
2.16 mol of lithium fluoride and 0.395 mol of fluorine