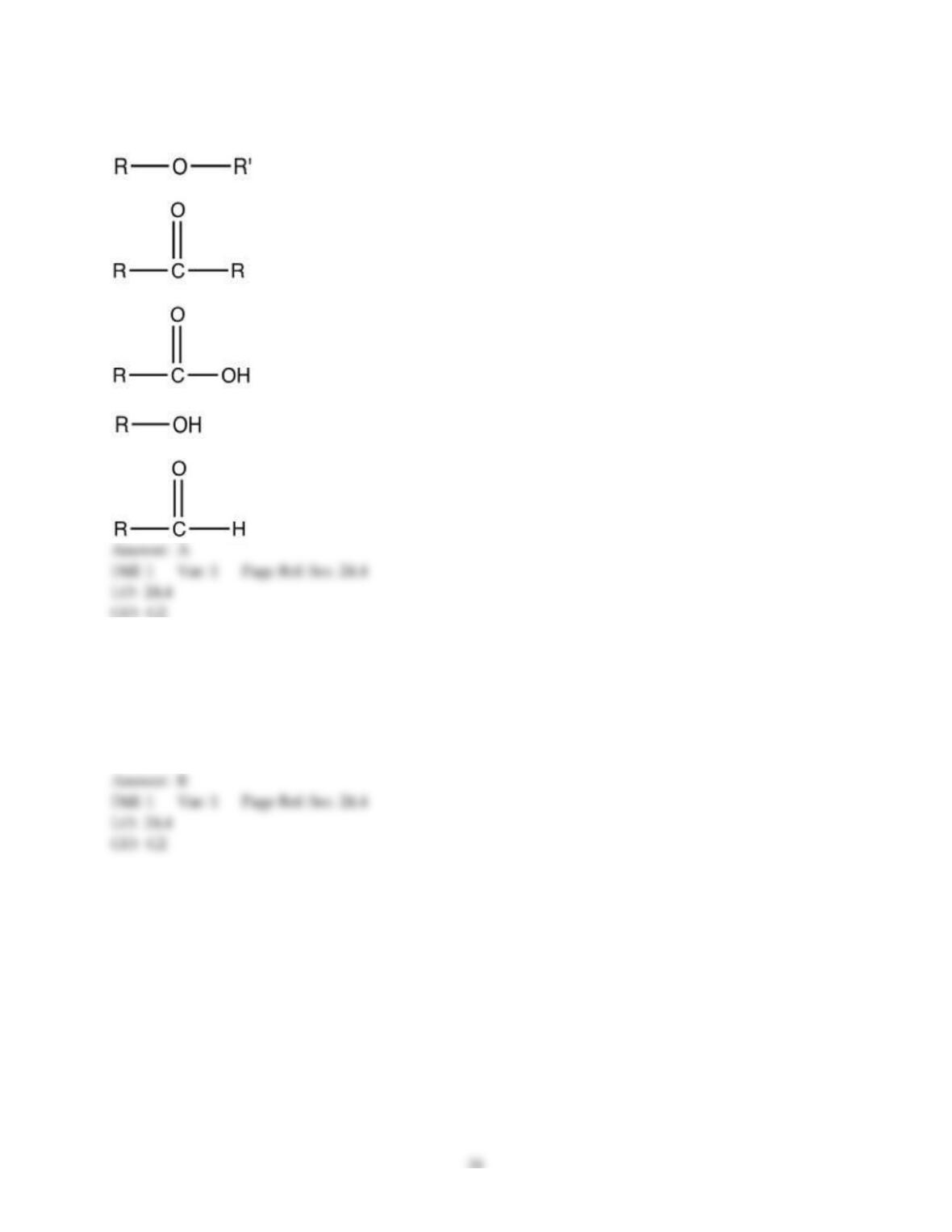
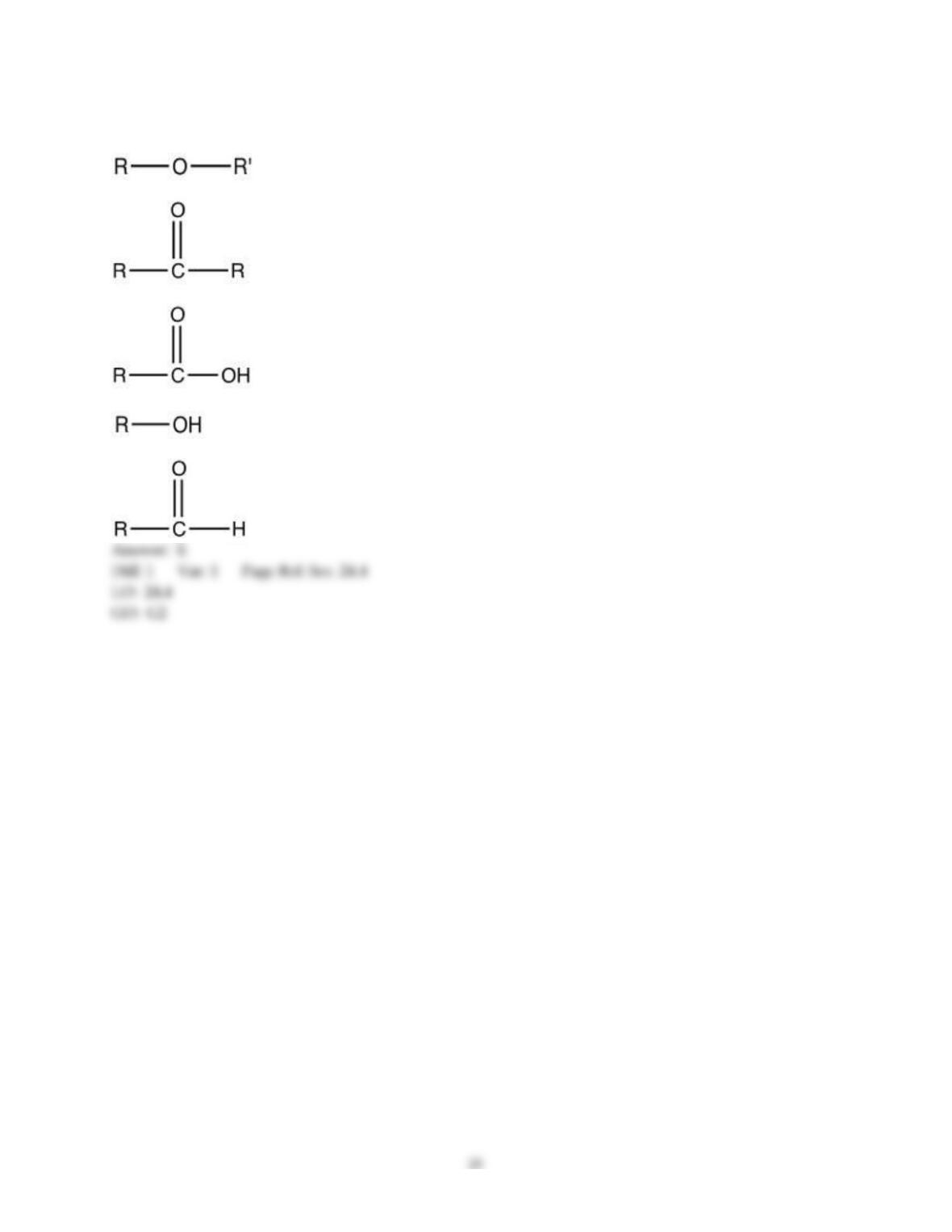
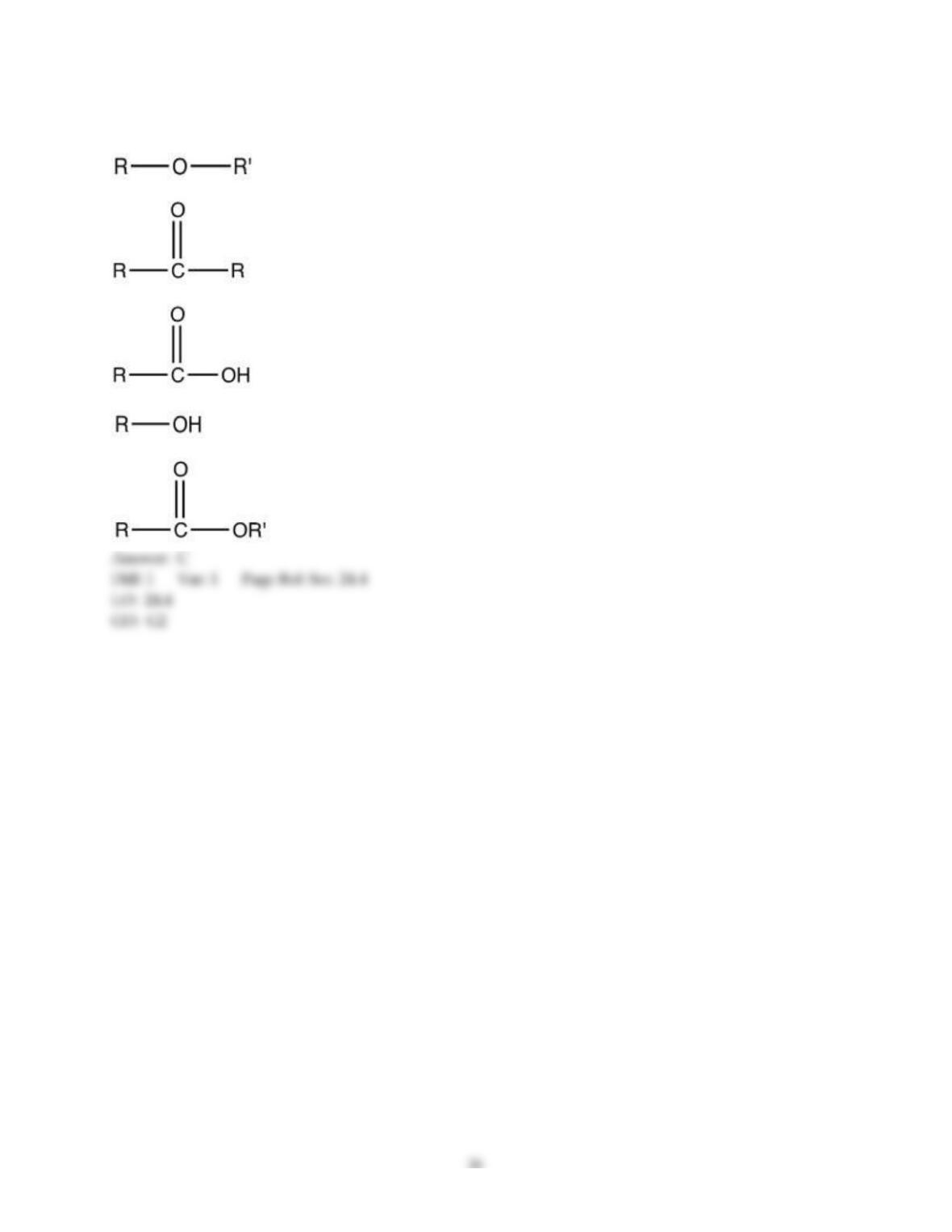
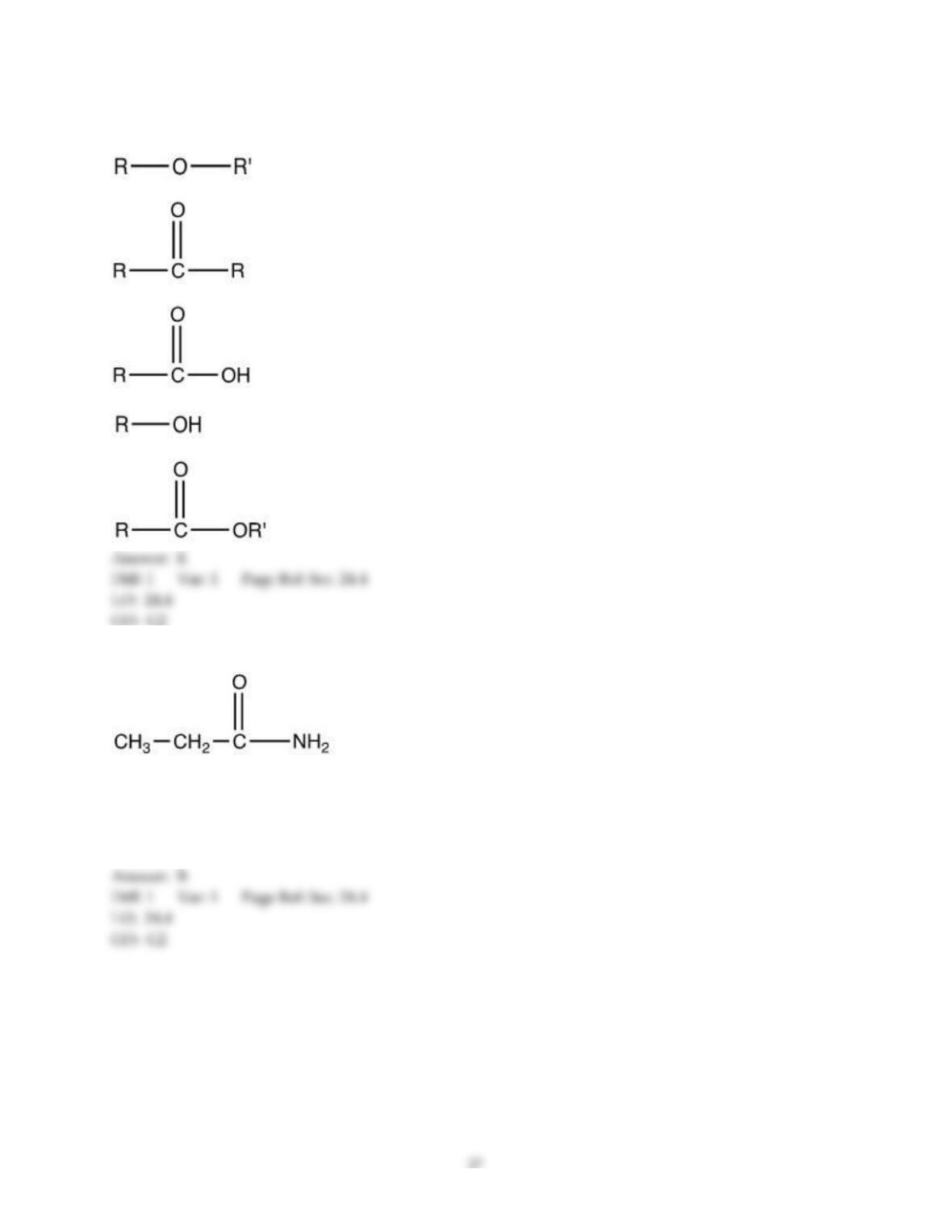
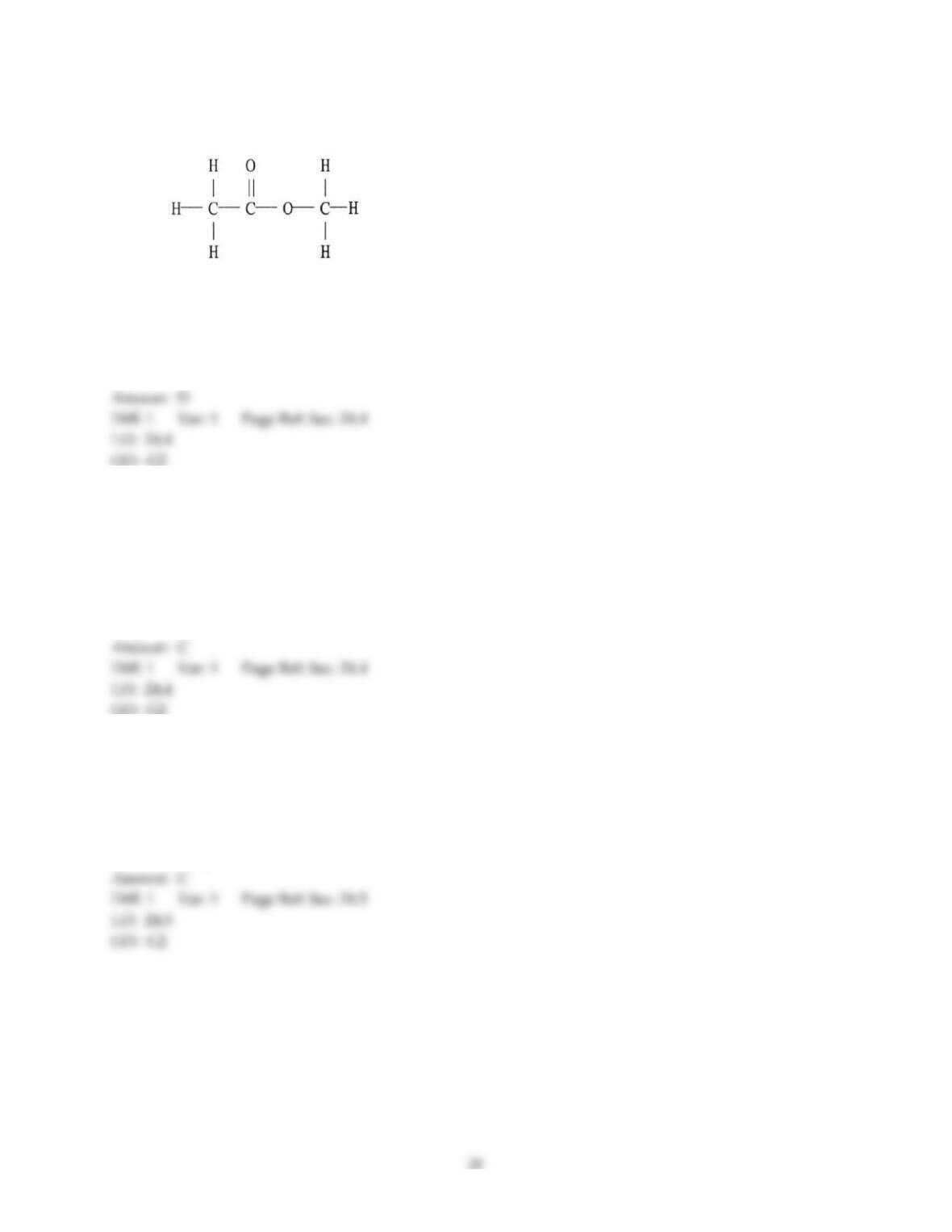
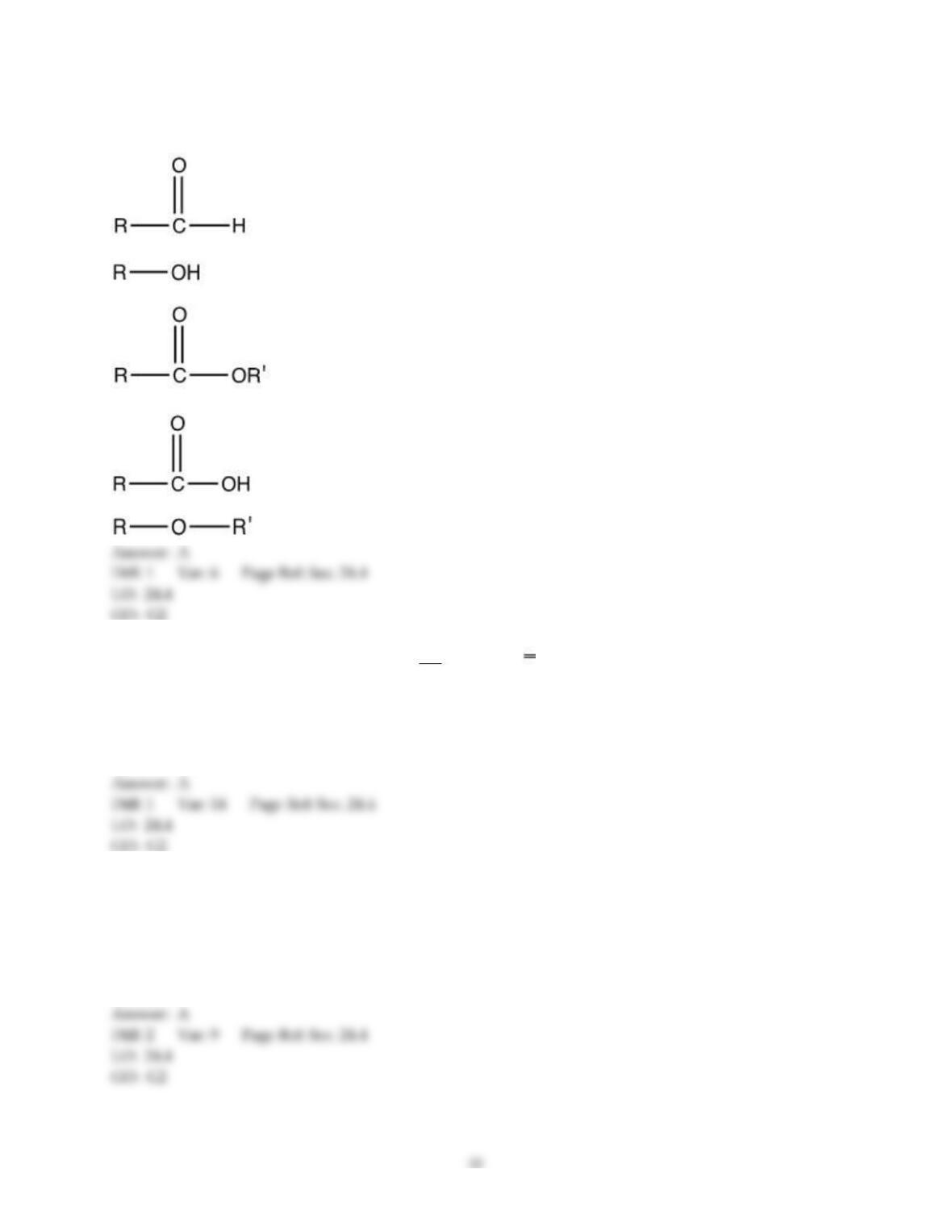
18) The aromas of different fruit are due to the chemical compounds known as ________.
19) The hydrolysis of an ester in the presence of a base is called ________.
20) Non-superimposable mirror-image isomers of a substance are called ________.
21) Living organisms must expend energy to counter any increase in ________.
22) The doubly ionized form of an amino acid is called a(n) ________.
23) Of the 20 amino acids found in our bodies, ________ of them must be ingested because our bodies
cannot synthesize sufficient quantities of them.
24) Large protein molecules that act as catalysts are called ________.
25) The most important acidic and basic functional groups in all amino acids are the ________ and the
________ groups, respectively.