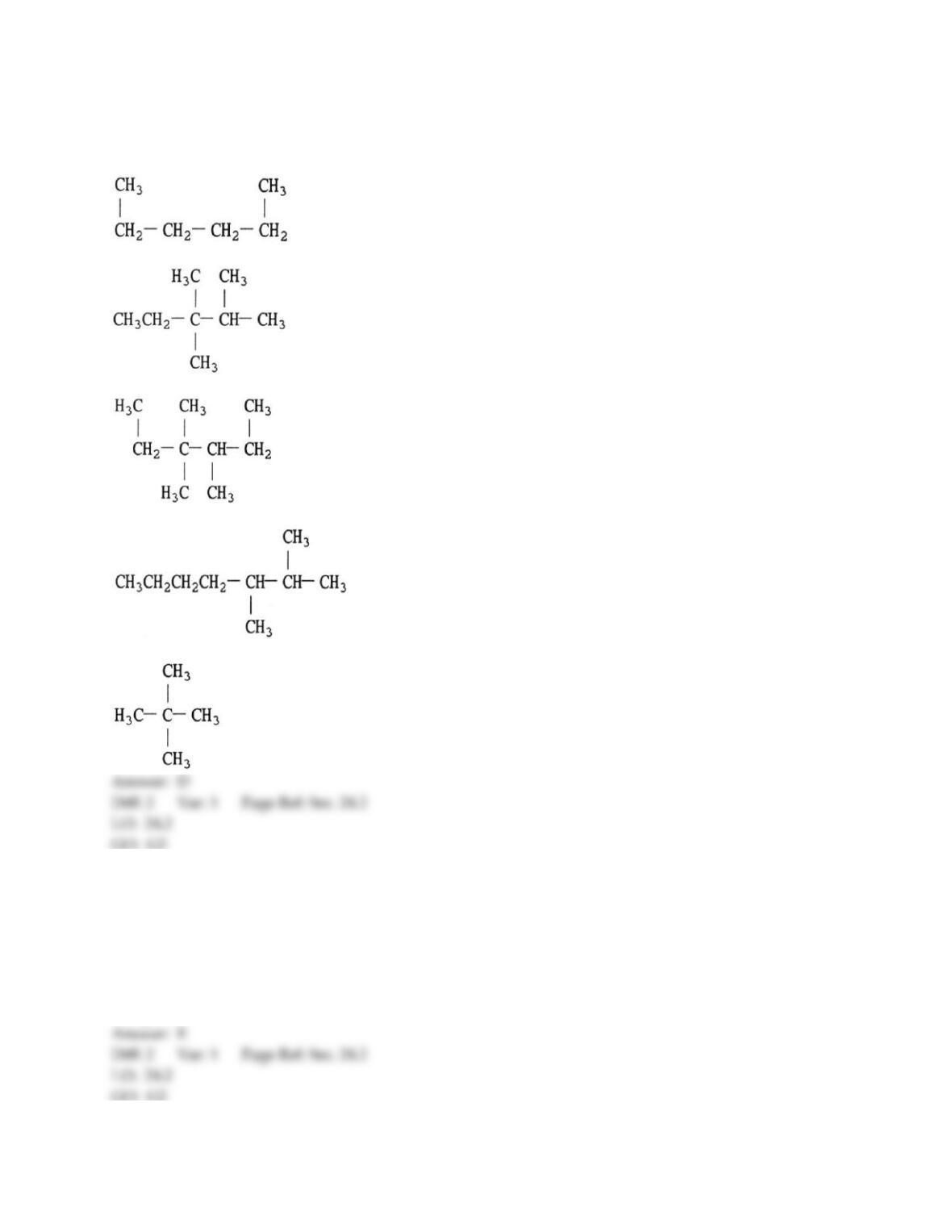
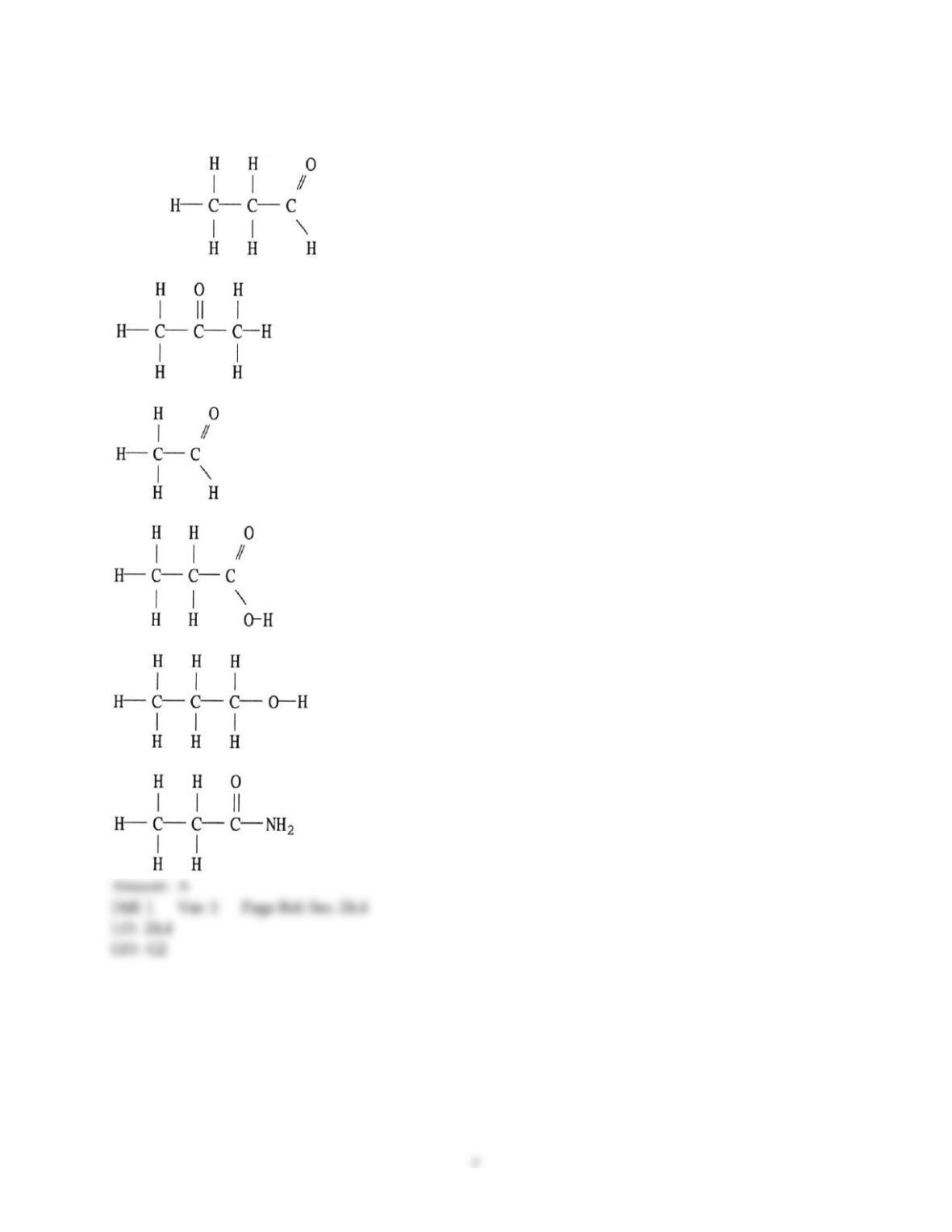
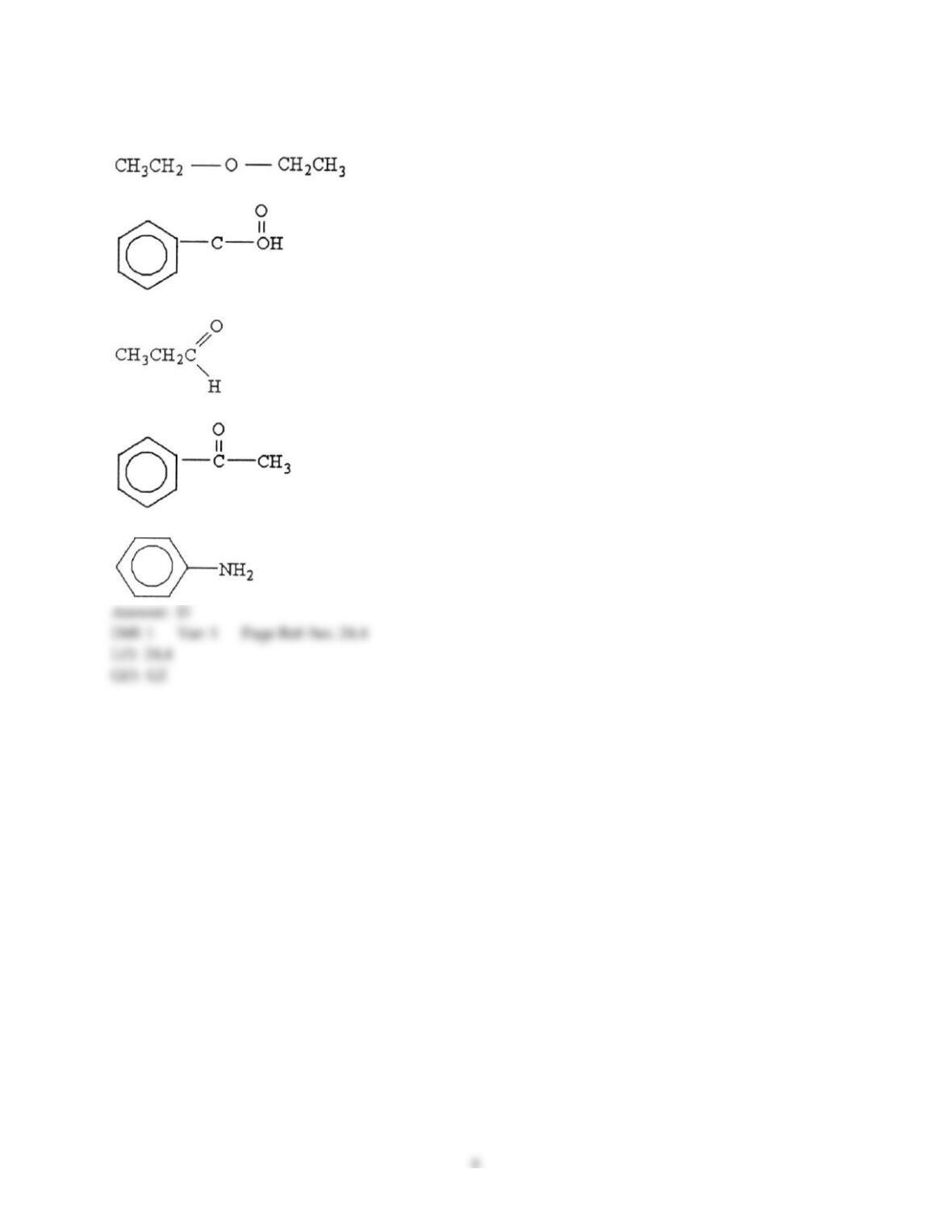
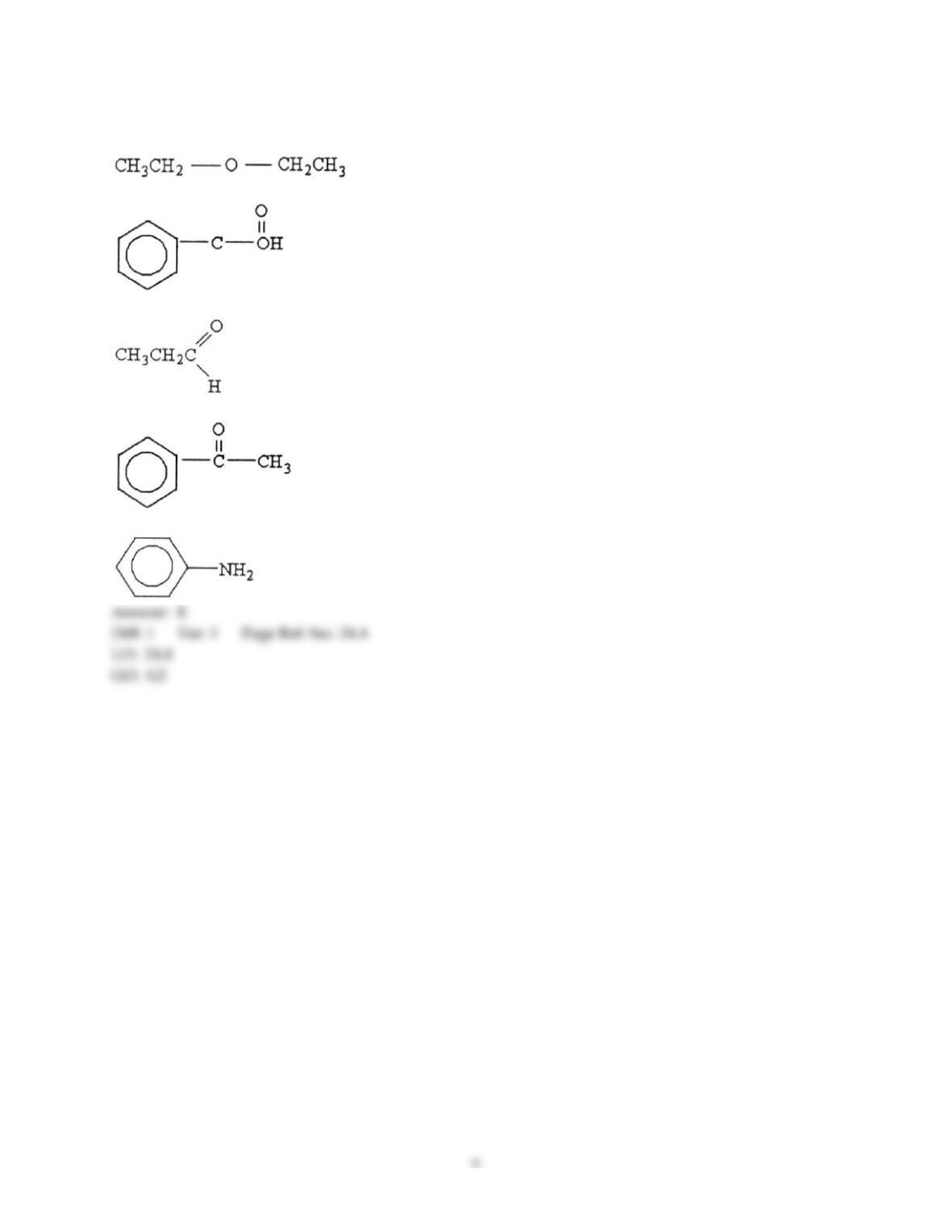
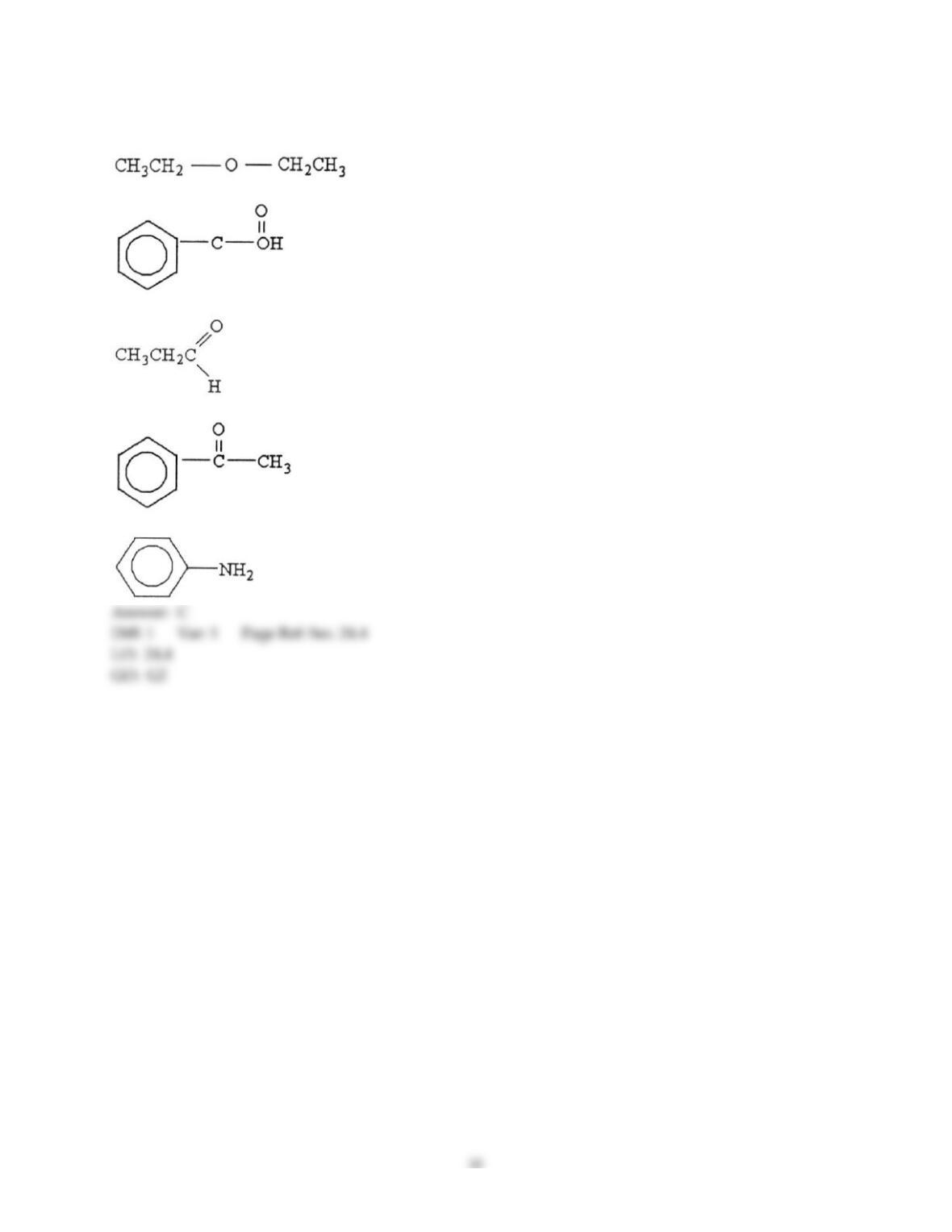
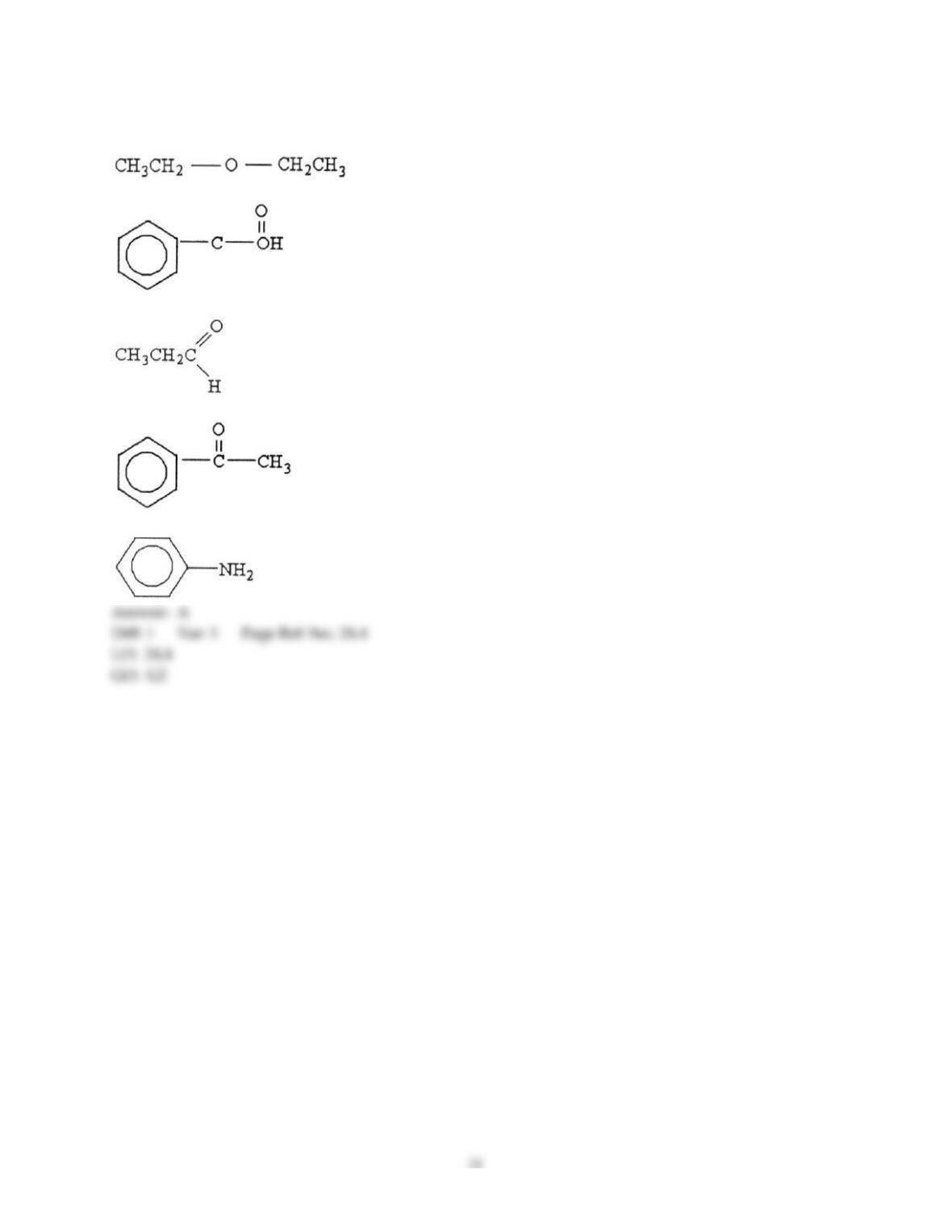
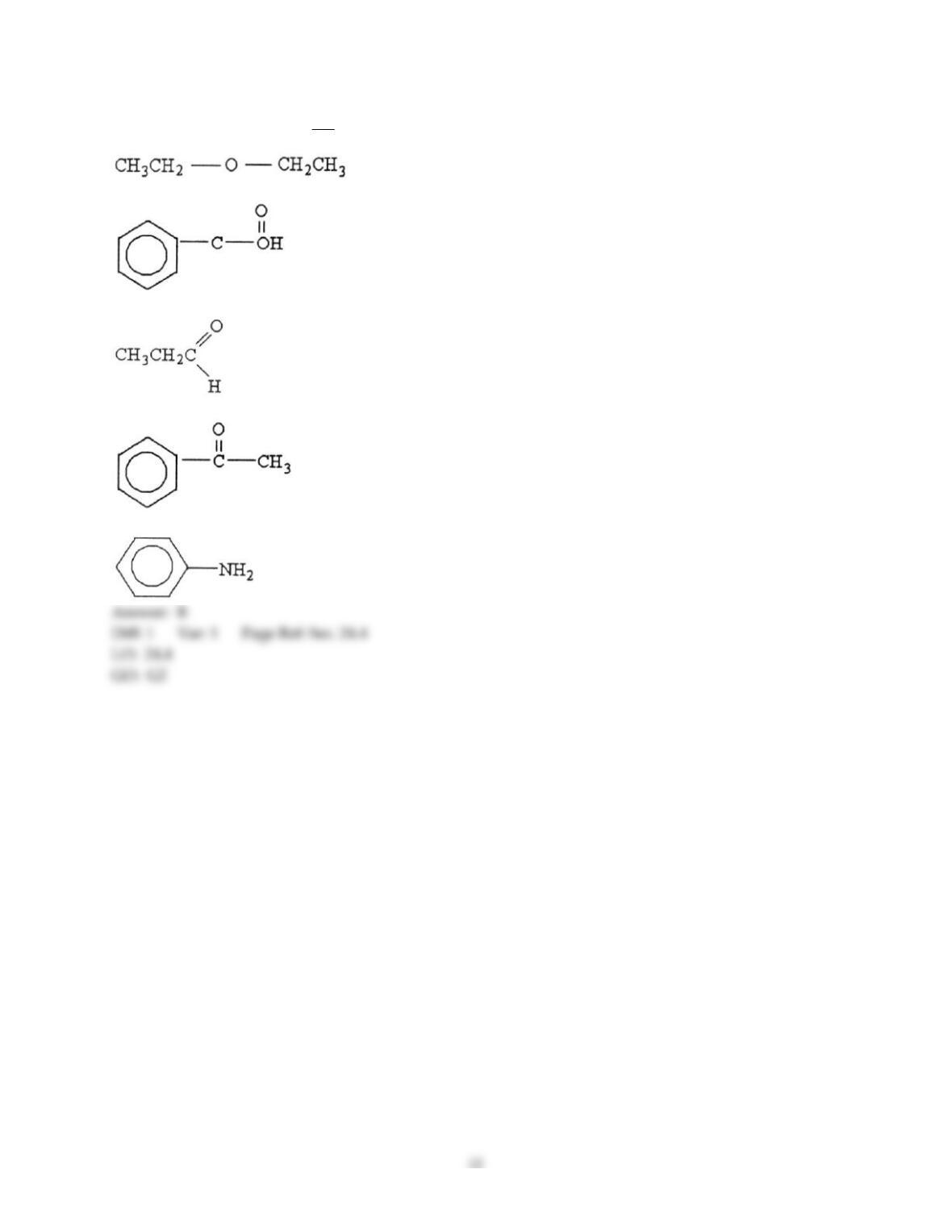
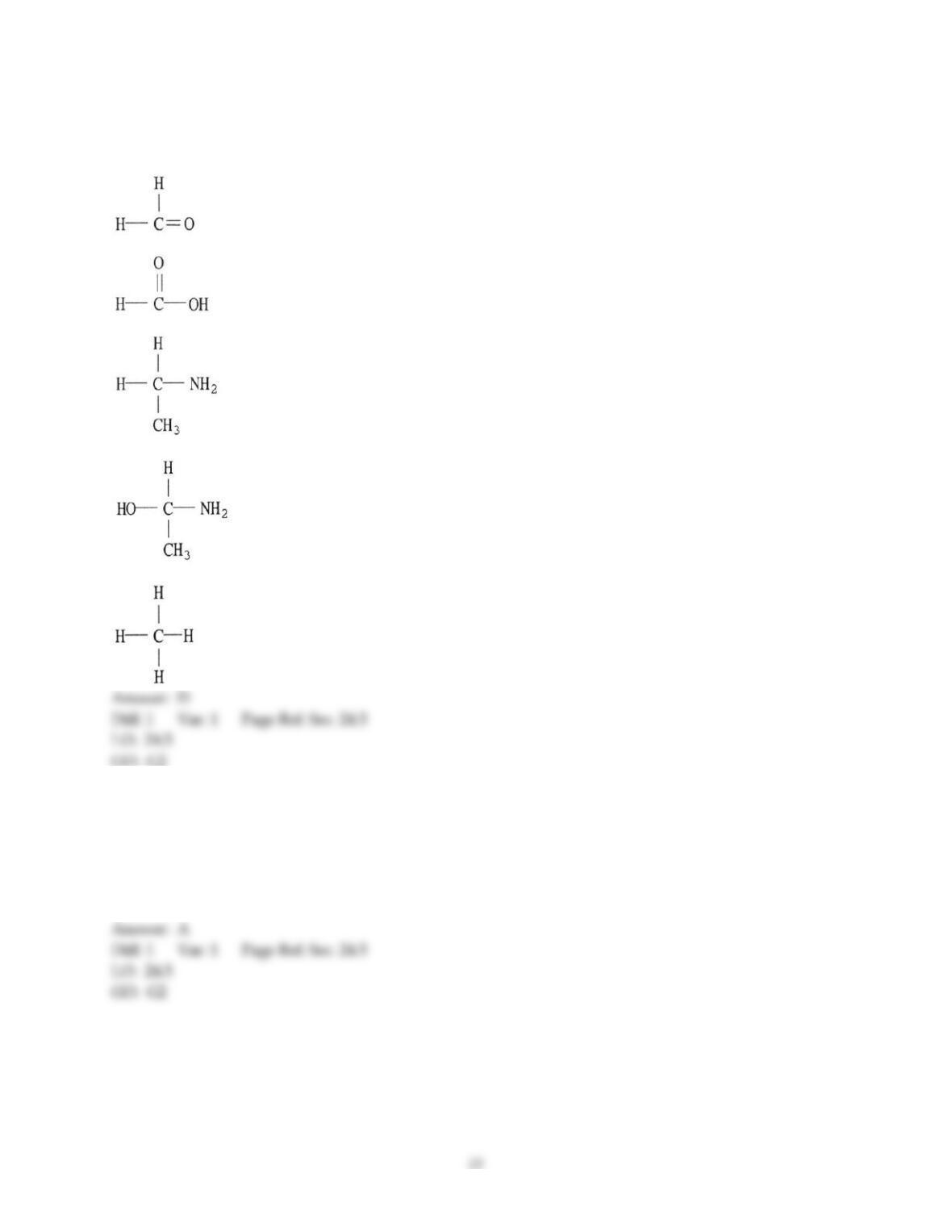
12) Which statement about addition reactions between alkenes and HBr is false?
A) The addition occurs at the double bond.
B) Bromine attacks the alkene carbon atom possessing a partial positive charge.
C) A hydrogen atom attaches to the alkene carbon atom possessing a partial negative charge.
D) The π bond breaks in the course of the reaction.
E) The proposed mechanism involves radicals.
13) Benzene behaves differently from a hydrocarbon which simply contains three C C bonds in that the
latter would be expected to react much more readily with ________.
A) H2
B) Cl2
C) Br2
D) HCl
E) all of the above
14) Alcohols are hydrocarbon derivatives in which one or more hydrogens have been replaced by a
hydroxyl functional group. ________ is the general formula of an alcohol.
A) R—O—R
B) R—CO—R
C) R—CO—OH
D) R—OH
E) R—CO—H
15) Which one of the following is not an alcohol?
A) acetone
B) glycerol
C) ethanol
D) cholesterol
E) ethylene glycol