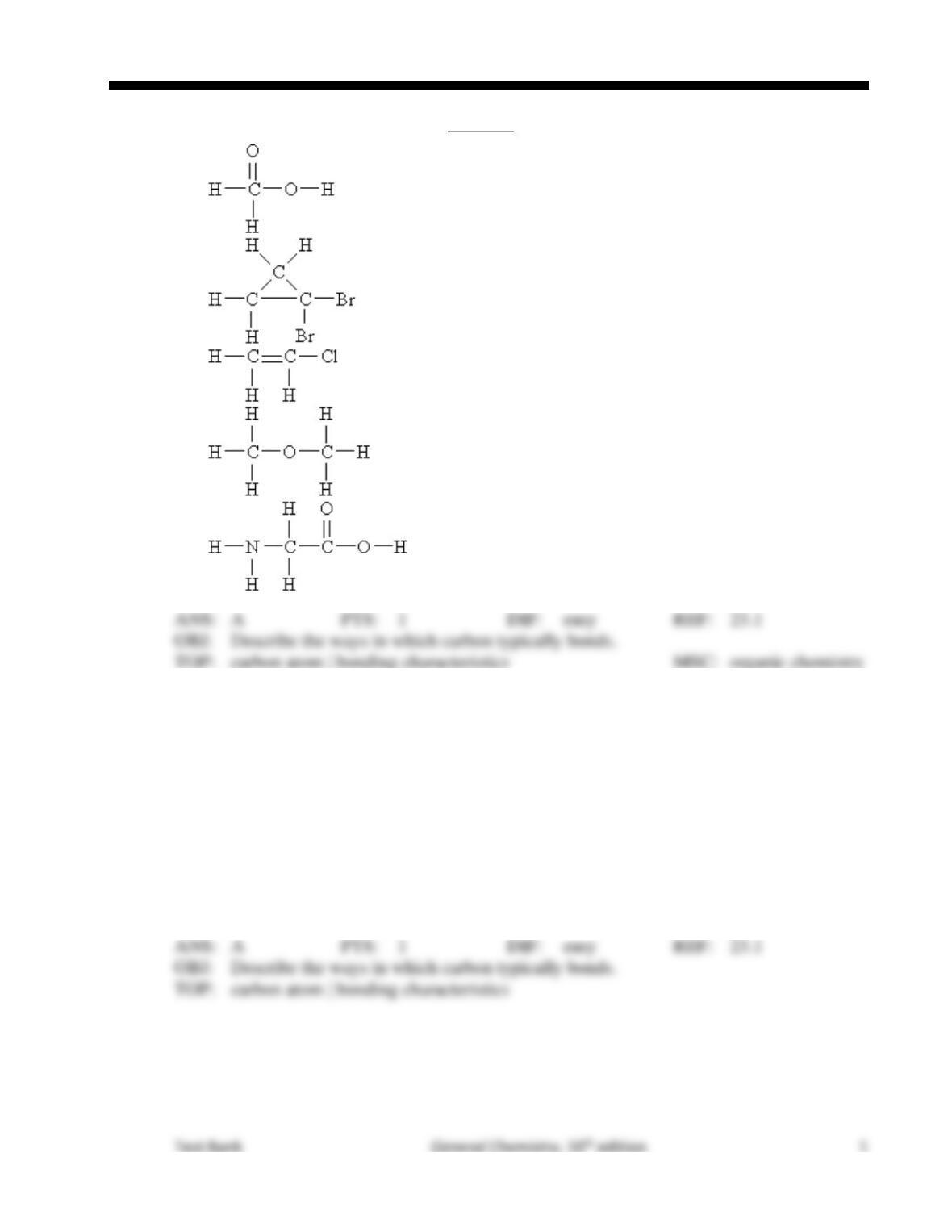
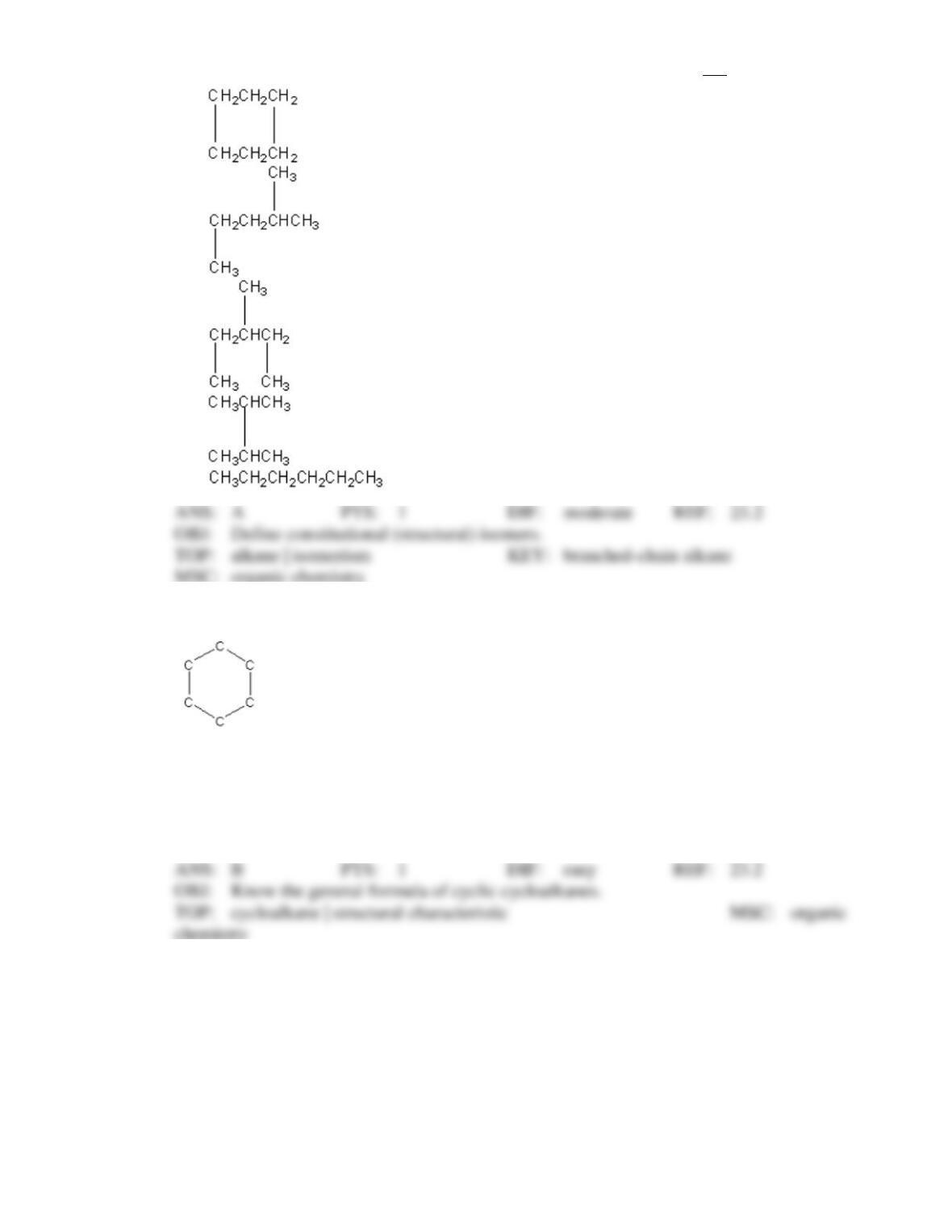
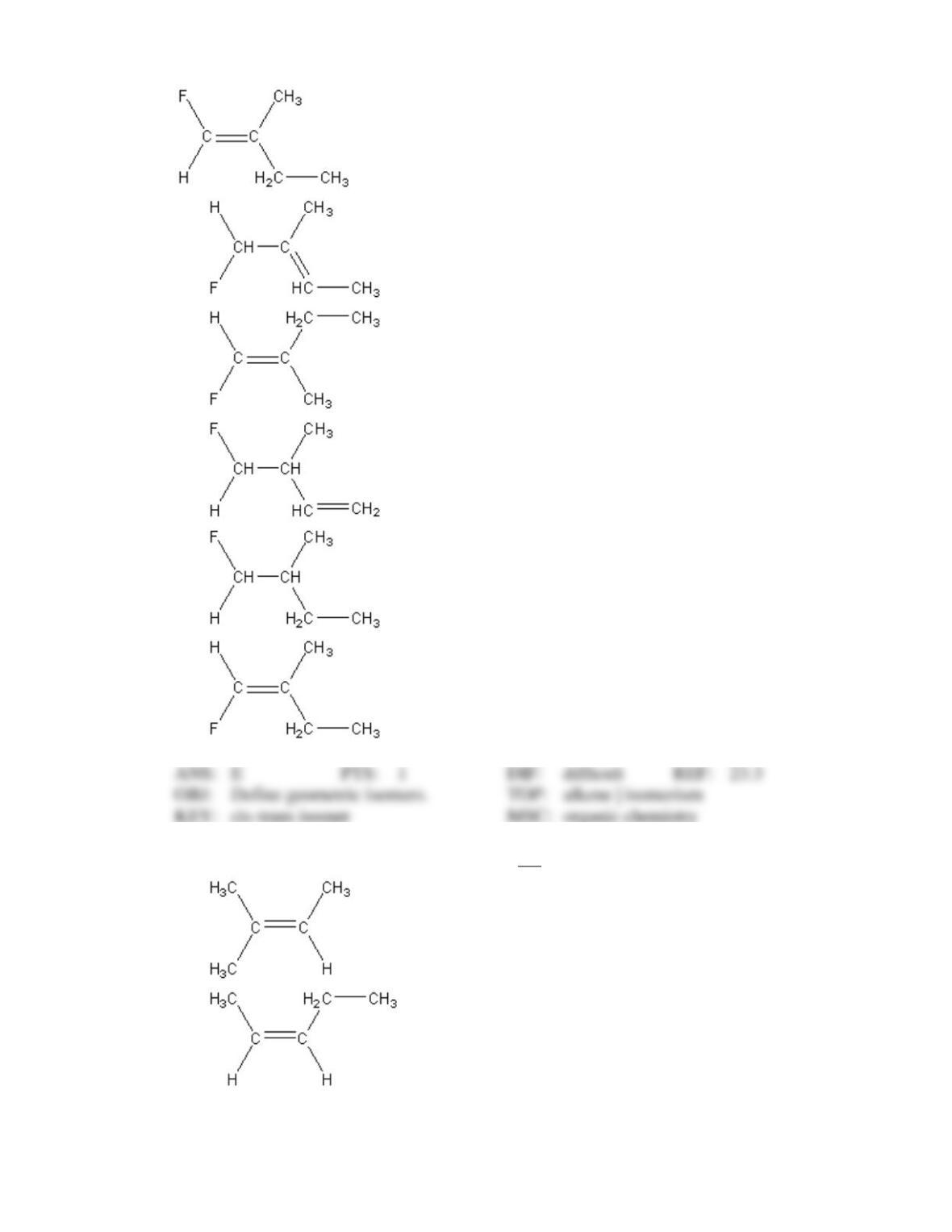
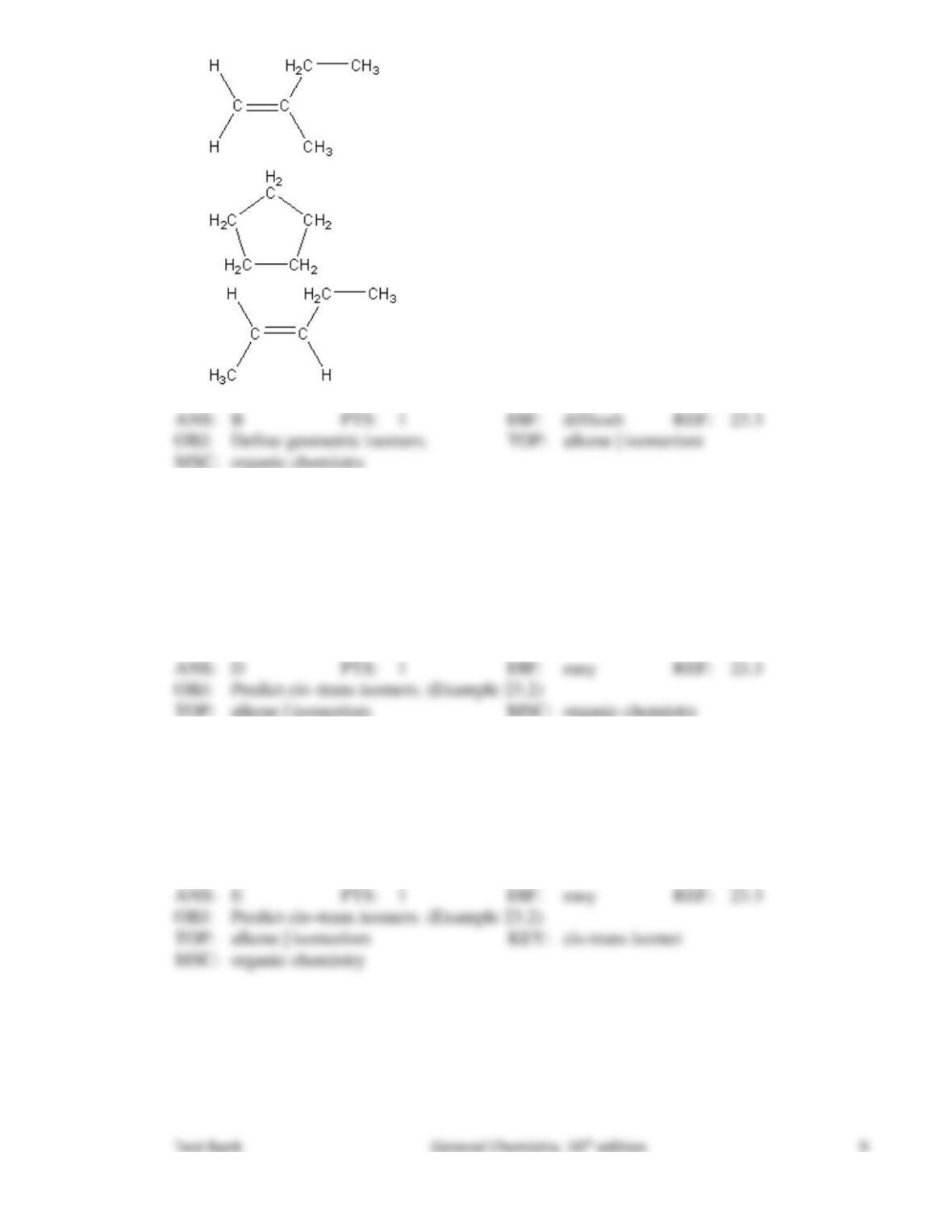
Test Bank General Chemistry, 10th edition 6
16. What is the composition of crude oil?
mostly unsaturated hydrocarbons having 5–12 carbons per molecule
mostly hydrocarbons, but the precise composition varies widely depending on
location
mostly aromatic hydrocarbons
mostly saturated hydrocarbons having 5–12 carbons per molecule
mostly saturated hydrocarbons having 12–20 carbons per molecule
17. Equal masses of each of the following are reacted with an excess of dioxygen. Which would
form the greatest amount of carbon dioxide?
Two of these would form equal amounts.
All of these would form equal amounts.
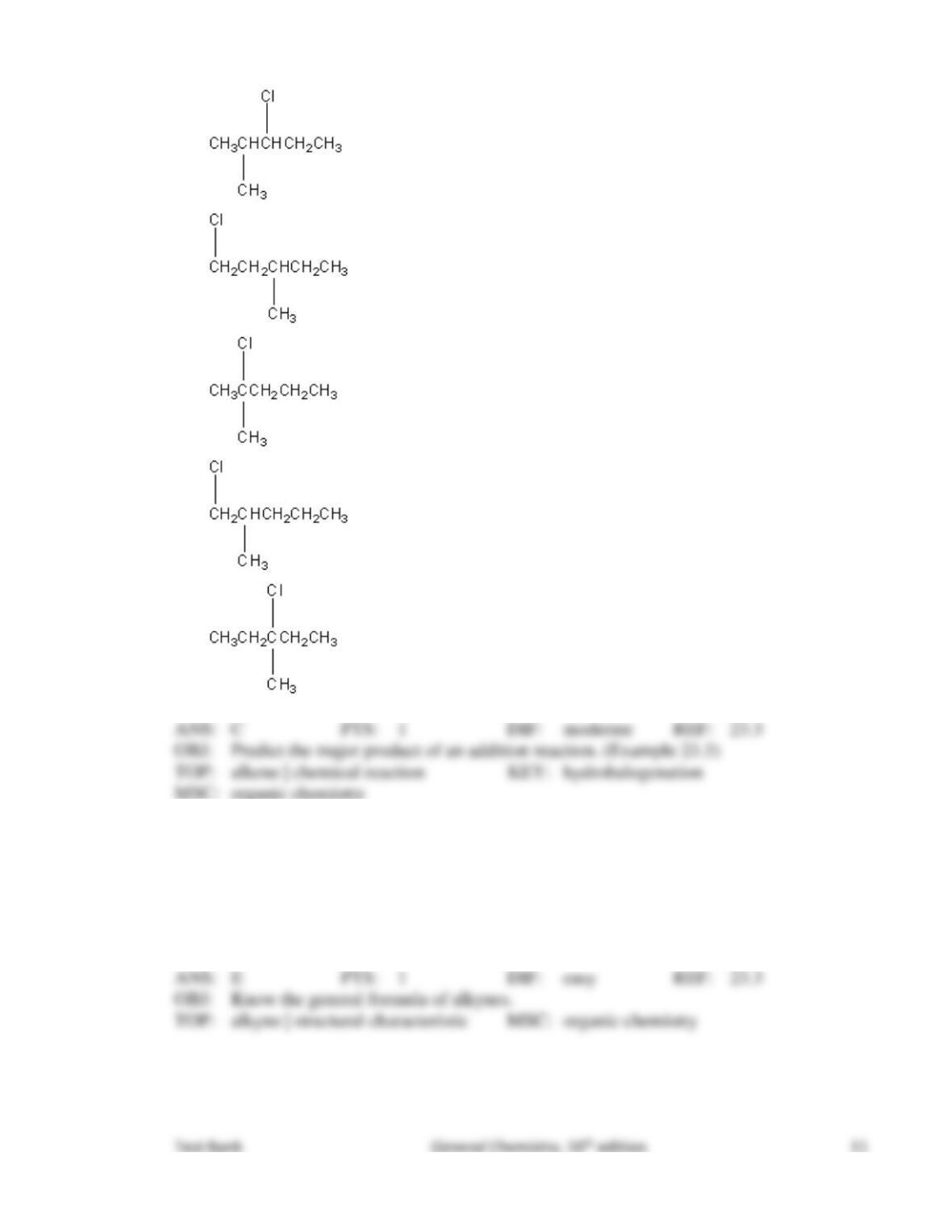
18. Which of the following is a possible product of the chlorination of butane in the presence of
light?
19. Which of the following chemical equations represents a substitution reaction?
HCOOH + CH3OH → HCOOCH3 + H2O
CH3OH + 3/2O2 → CO2 + 2H2O
C2H6 + Cl2 → CH3CH2Cl + HCl