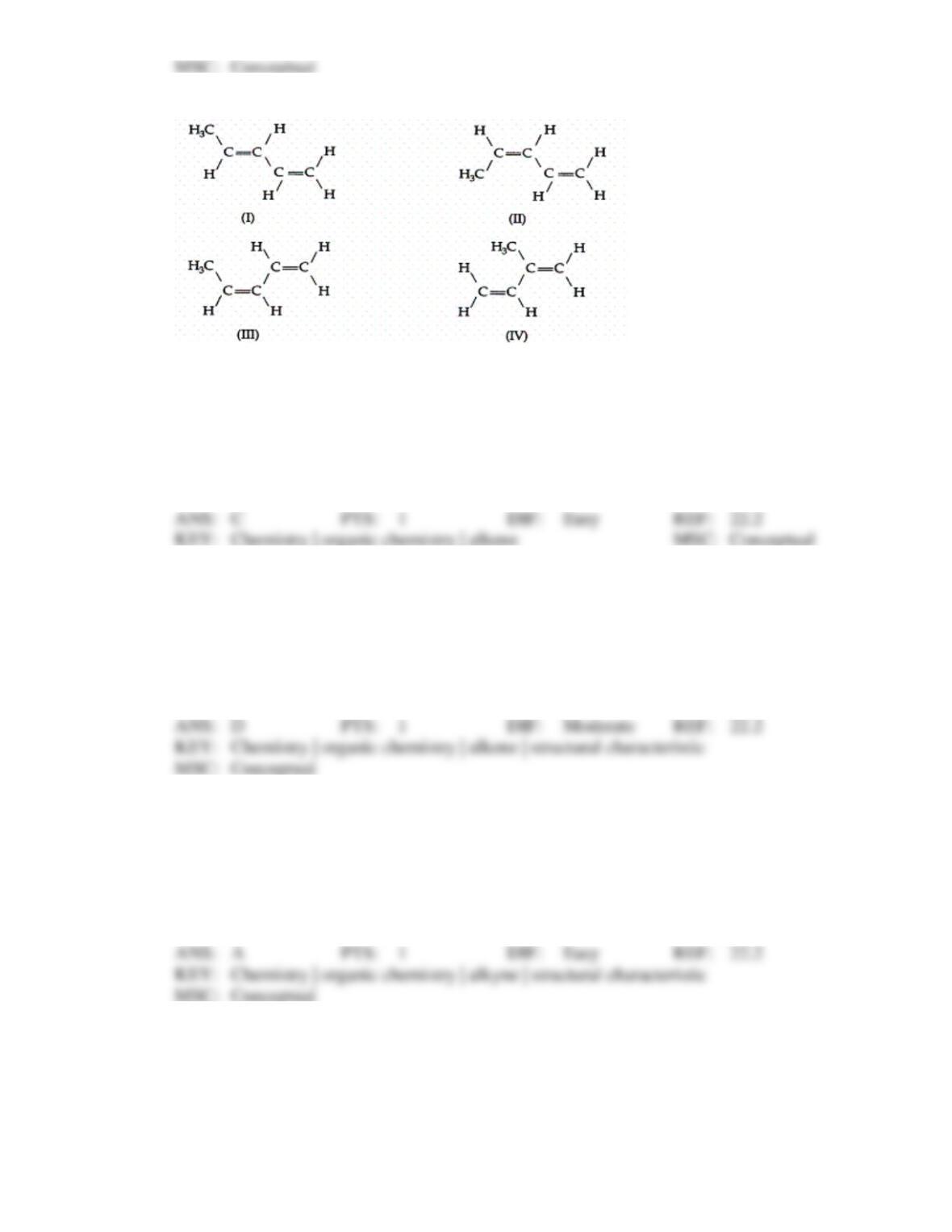
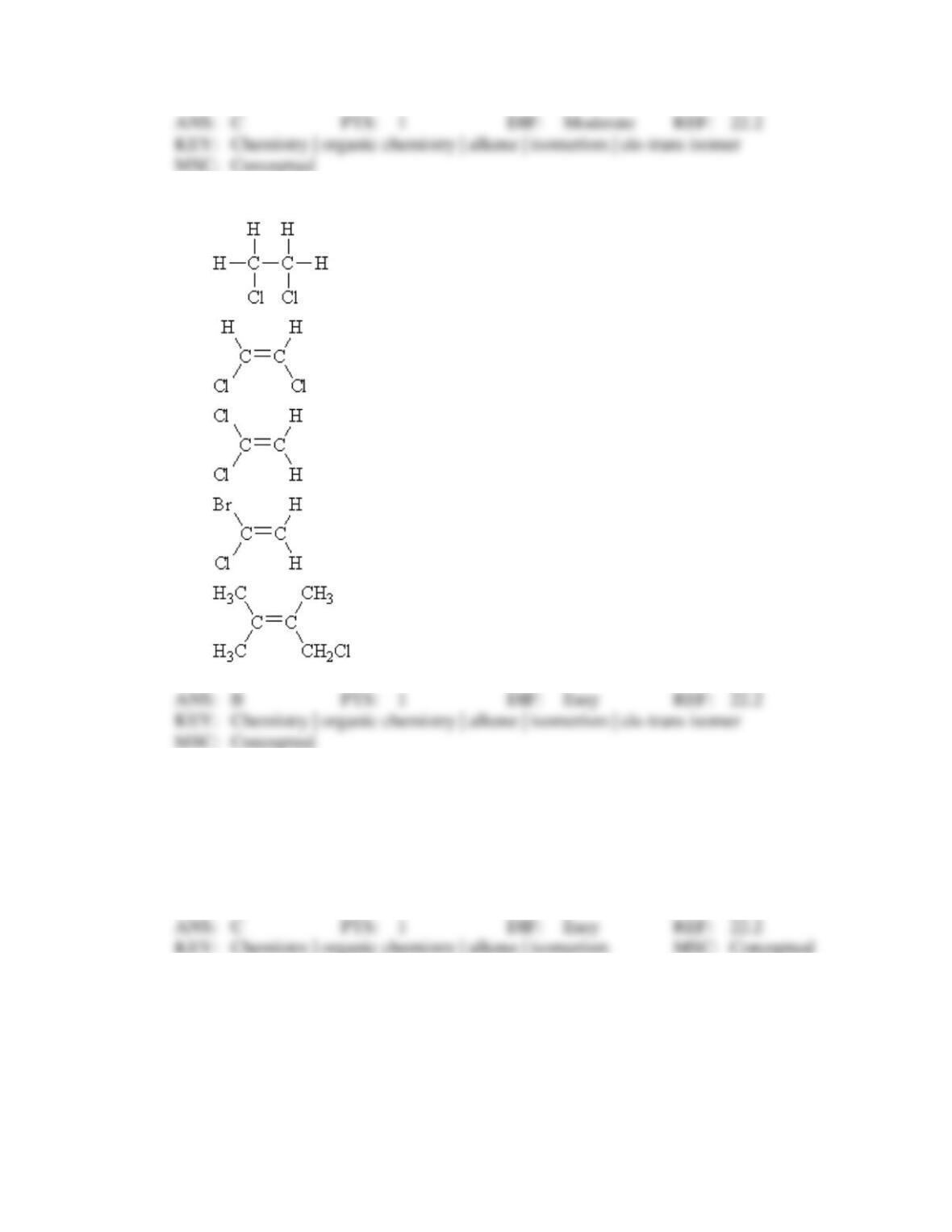
However, the teacher pointed out that, although the molecule could be correctly drawn from
this name, the name violates the IUPAC rules. What is the correct (IUPAC) name of the
molecule?
2-t-butyl-4-methylpentane
2,2,3,5-tetramethylhexane
2,4,5,5-tetramethylhexane
1-sec-butyl-1,2,2-trimethylpentane
8. Which of the following names is a correct one?
3-methyl-4-isopropylpentane
2-ethyl-4-tertiary-butylpentane
2,2,3,5-tetramethylheptane
9. What is the compound whose carbon skeleton (minus any hydrogen atoms) appears below?
2,4-diethyl-3,6-dimethylheptane
2,5-dimethyl-4,6-diethylheptane
1,4-diethyl-3,6-dimethyl-tridecane
5-ethyl-3,6-trimethyloctane
4-ethyl-2,5,6-trimethyloctane
10. A student gave a molecule the following name: 2-ethyl-3-methyl-5-isopropylhexane
However, his TA pointed out that, although the molecule could be correctly drawn from this
name, the name violates the systematic rules. What is the correct (systematic) name of the
molecule?
3,4-dimethyl-6-isopropylheptane
2-isopropyl-4,5-dimethylheptane
3,4,6,7-tetramethyloctane
1,2-diethyl-3,6,7-trimethylheptane
2,3,5,6-tetramethyloctane