47. You analyze for pyridine (Kb is approximately 10–9) by dissolving 0.1000 g of complex in
10 mL of H2O and titrating with a 0.01 M HCl solution. Which of the following indicators
should be used to detect the endpoint? (Assume that the initial concentration of pyridine is
approximately 0.01 M.)
bromophenol blue, pH range of color change = 3.0–4.6
methyl red, pH range of color change = 4.8–6.0
bromothymol blue, pH range of color change = 6.0–7.6
thymol blue, pH range of color change = 8.0 –9.6
alizarin yellow, pH range of color change = 10.1–12.0
48. Addition of AgNO3 to aqueous solutions of the complex results in a cloudy white
precipitate, presumably AgCl. You dissolve 0.1000 g of the complex in H2O and perform a
precipitation titration with 0.0500 M AgNO3 as the titrant. Using an electrode that is
sensitive to [Ag+], you reach the endpoint after 9.00 mL of titrant are added. How many
grams of chloride ion were present in the 0.1000-g sample?
49. Analysis of the data from a titration indicates that a 0.1190-g sample of the complex
contains 0.0844 g of py. Further analysis shows that 0.1190 g of the complex contains a
0.0157 g of cobalt and 0.0189 g of chloride. What is the empirical formula of the complex?
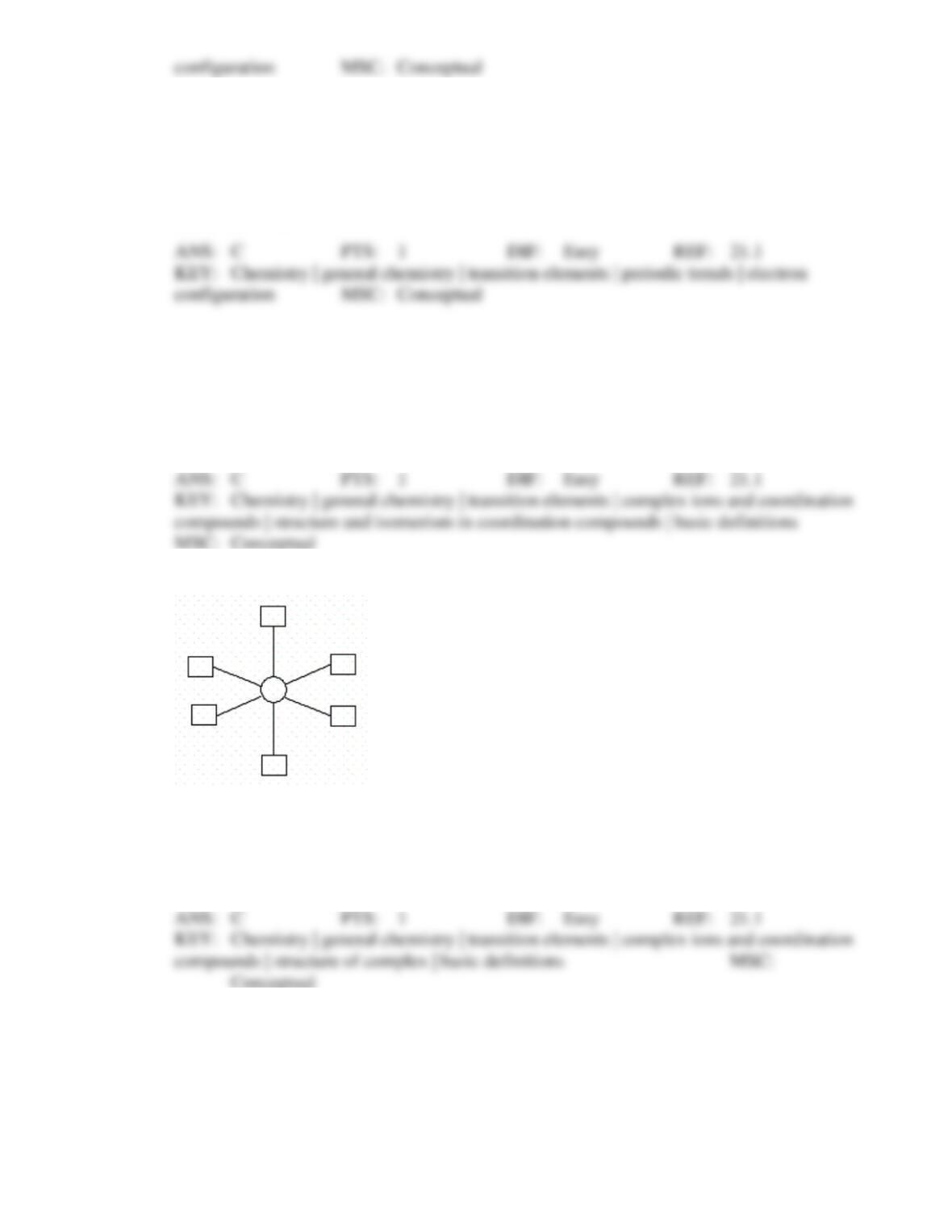
50. In which of the following complexes does the transition metal have a d8 configuration?