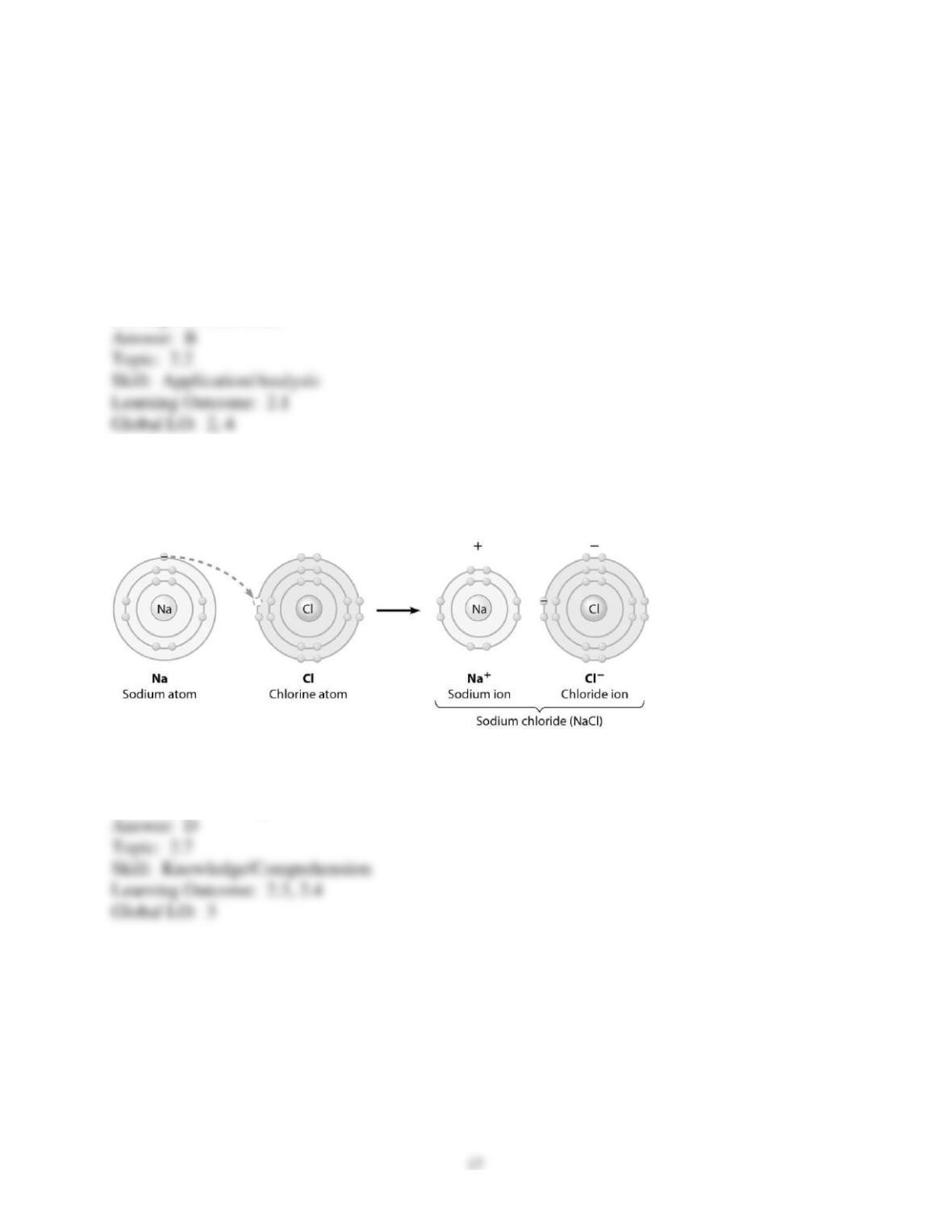
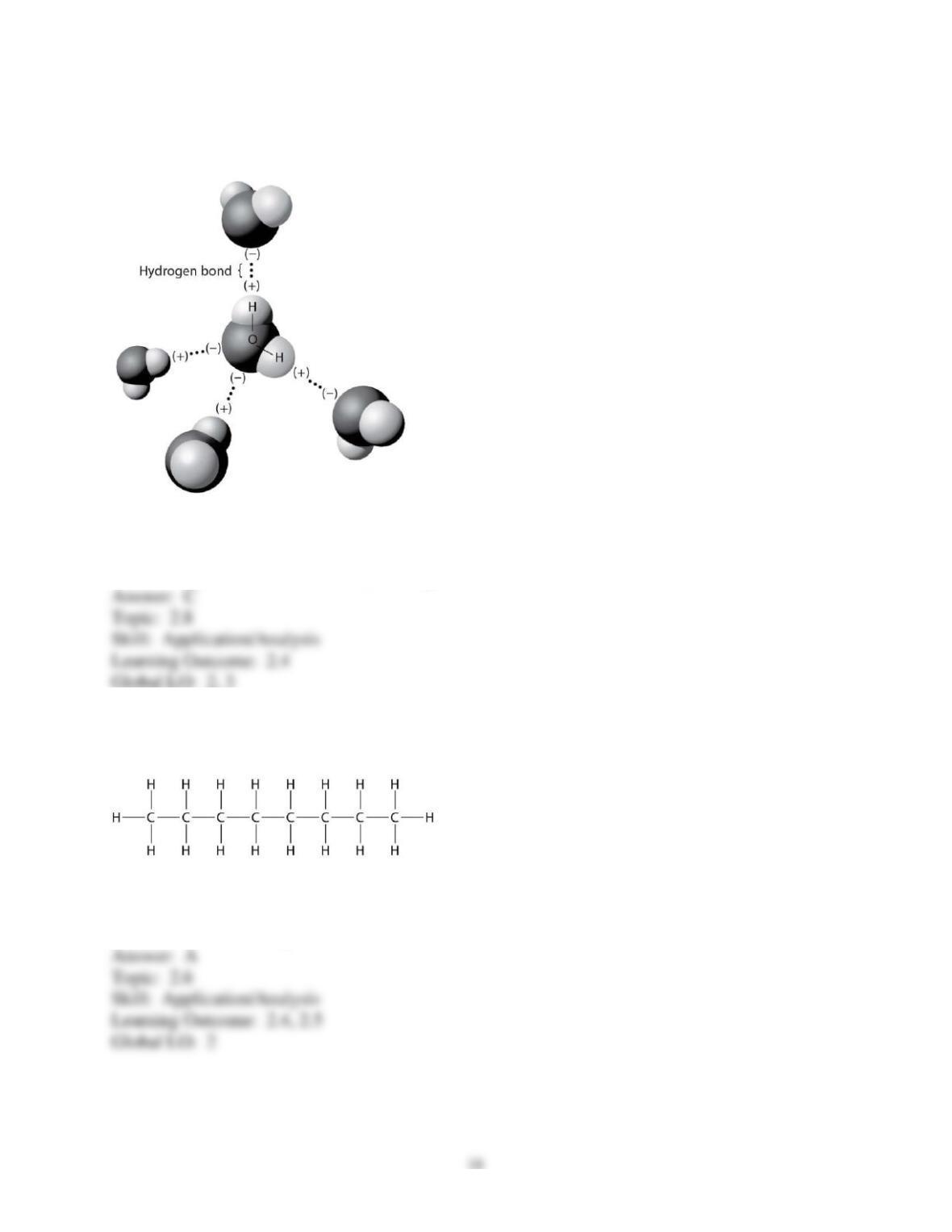
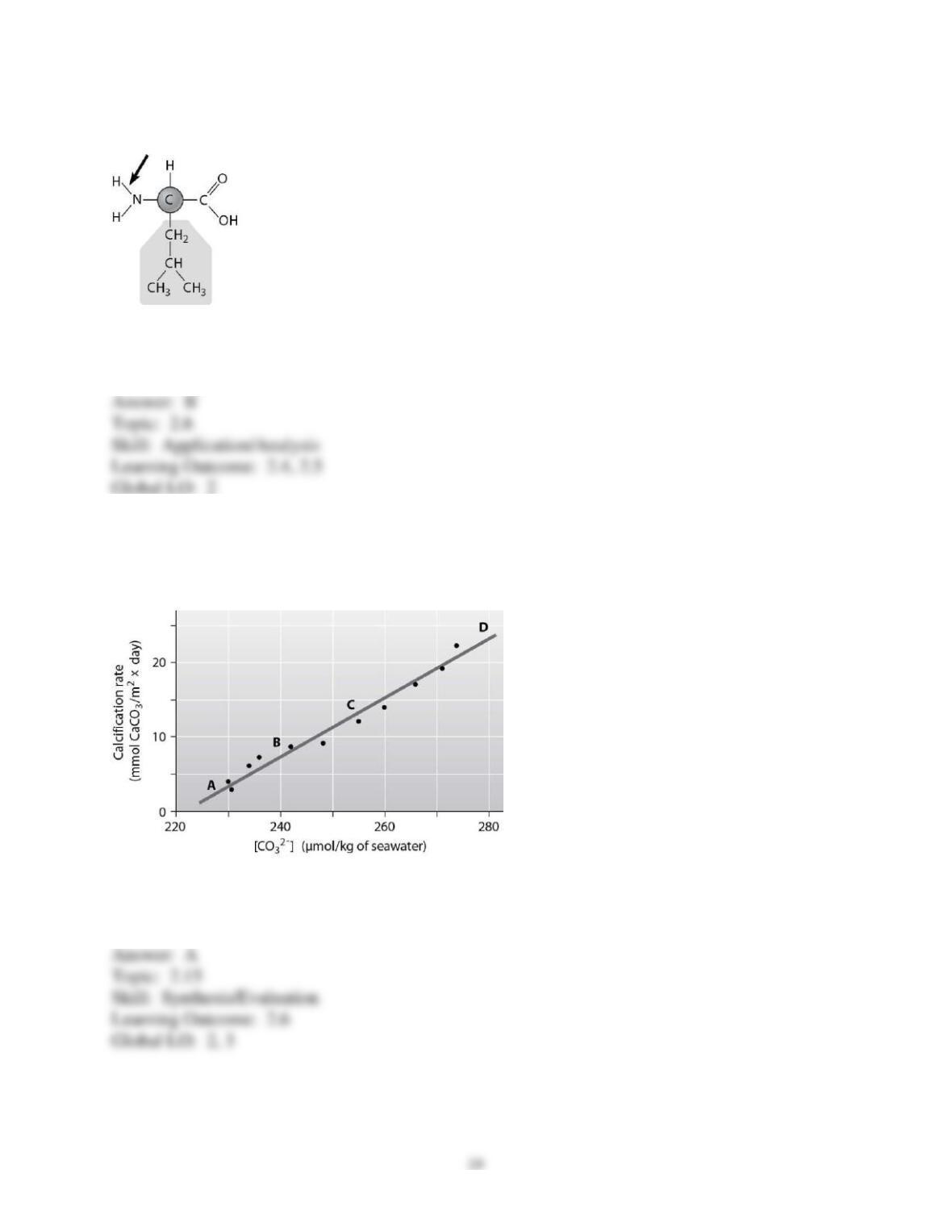
3) You want to design a controlled experiment to determine which tablet, PEPCID AC or Alka-
Seltzer, neutralizes stomach acid the quickest. Which of the following experiments would be the
best to perform?
A) Place a PEPCID AC tablet in a beaker of acid at pH 2 and an Alka-Seltzer tablet in a separate
beaker of acid at pH 2 and check the pH in the beakers every 2 minutes.
B) Take a PEPCID AC tablet at 8:00 AM on Monday and take an Alka-Seltzer tablet at 8:00 AM
on Tuesday and take note of how long it takes you to feel better.
C) Find a group of your friends and go out to a big dinner. Then give half of them a PEPCID AC
tablet and give the other half an Alka-Seltzer tablet and tell them to write down how long it takes
them to feel better.
D) Place a PEPCID AC tablet in a beaker of acid at pH 2 and an Alka-Seltzer tablet in a separate
beaker of acid at pH 2 and record how long it takes for the tablets to dissolve.
4) You hypothesize that a tablet of PEPCID AC can neutralize more stomach acid than a tablet
of Alka-Seltzer. If you placed a tablet of each antacid in a beaker of acid at an initial pH of 2,
which of the following experimental results would support your hypothesis?
A) After 1 hour, the pH of the solution in the PEPCID AC beaker was 9.2 and the pH of the
solution in the Alka-Seltzer beaker was 8.3.
B) After 1 hour, the pH of the solution in the PEPCID AC beaker was 7.9 and the pH of the
solution in the Alka-Seltzer beaker was 9.6.
C) After 1 hour, the pH of the solution in the PEPCID AC beaker was equal to that of the pH of
the solution in the Alka-Seltzer beaker.
D) It took 3 minutes for the PEPCID AC tablet to fully dissolve and it took 5 minutes for the
Alka-Seltzer tablet to fully dissolve.
5) You want to design an experiment to compare the effectiveness of Pepcid AC tablets and
Alka-Seltzer tablets. Which of the following facts would be least likely to complicate your
experiment and the analysis of your results?
A) PEPCID AC and Alka-Seltzer tablets contain different concentrations of the antacid drug.
B) PEPCID AC and Alka-Seltzer tablets contain different ingredients.
C) The recommended dosage for PEPCID AC and Alka-Seltzer tablets is different.
D) PEPCID AC and Alka-Seltzer tablets are sold in different countries throughout the world.