For each of the following situations, choose the correct type of chemical bond.
nonpolar covalent bond(s)
84. Occurs when electrons are shared equally between two atoms
85. Used by geckos for clinging to and climbing up smooth vertical surfaces
86. Formed by the attraction between partial positive and partial negative charges created by unequal
electron sharing
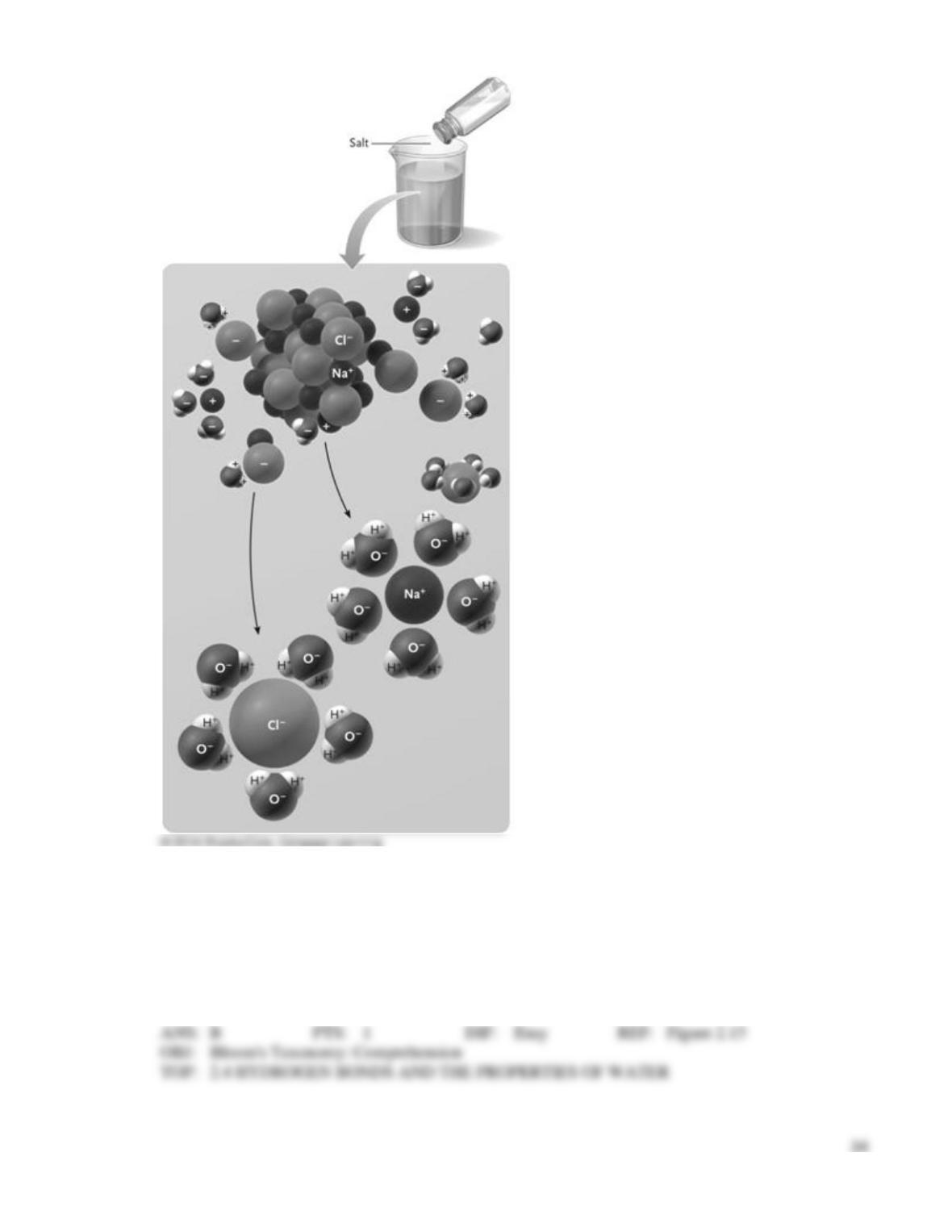
87. Occurs in sodium chloride (NaCl)
88. The weakest of the chemical linkages listed
89. Occurs in a water molecule (H2O)
90. Characteristic of molecules that contain atoms of only one kind
91. Forms when atoms gain or lose valence electrons completely
92. Attraction that arises when the constant movement of electrons, by chance, produces temporary zones
of partial positive and partial negative charges
93. Occurs when electrons are shared unequally between two atoms
94. Creates a region that is hydrophobic
95. Occurs between water molecules
96. Occurs in molecular oxygen (O2)