Test Bank General Chemistry, 10th edition 35
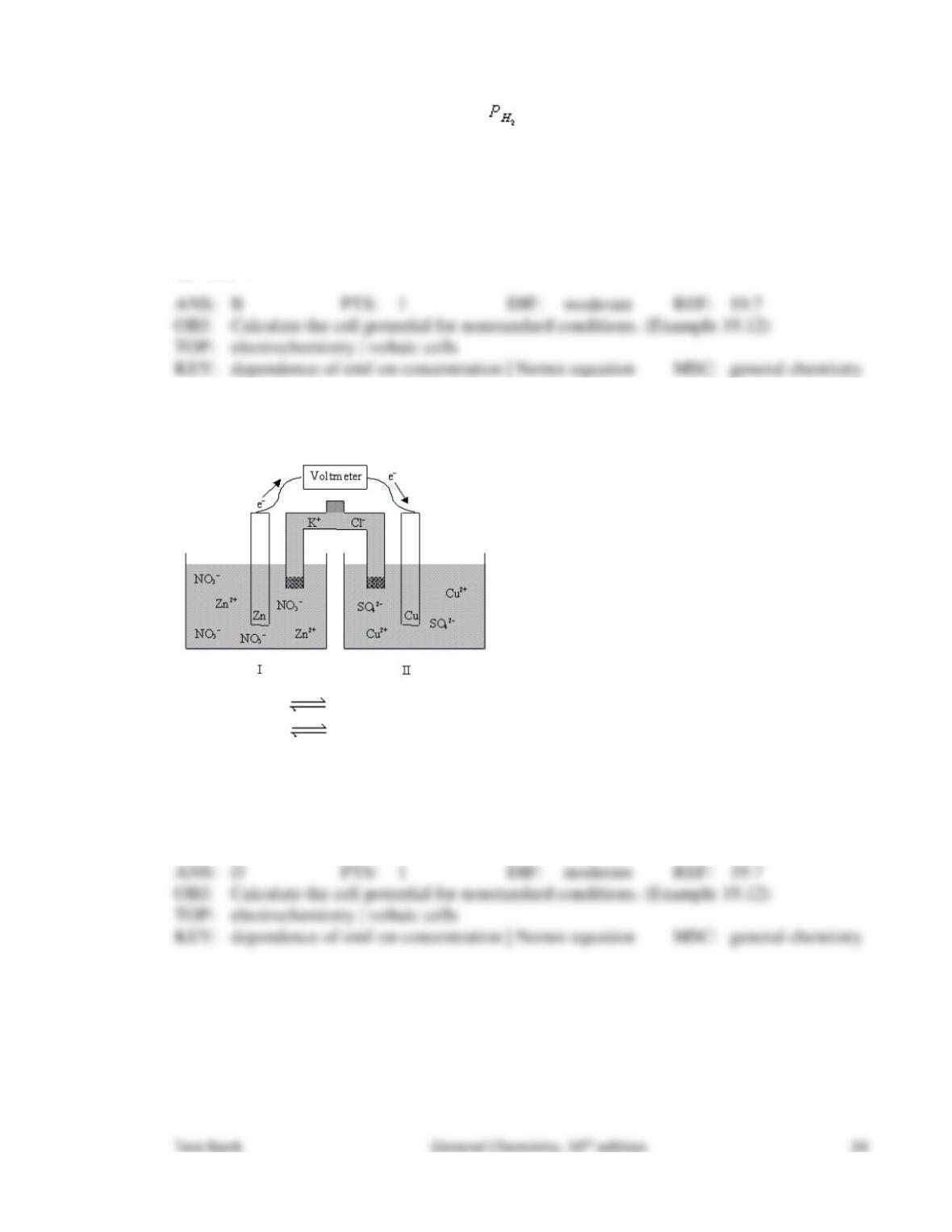
96. In order to determine the identity of a particular transition metal (M), a voltaic cell is
constructed at 25°C with the anode consisting of the transition metal as the electrode
immersed in a solution of 0.0977 M M(NO3)2, and the cathode consisting of a copper
electrode immersed in a 1.00 M Cu(NO3)2 solution. The two half-reactions are as follows:
M(s) M2+(aq) + 2e–
Cu2+(aq) + 2e– Cu(s)
The potential measured across the cell is 0.77 V. What is the identity of the metal M?
97. What is the reduction potential for the half-reaction Al3+(aq) + 3e– Al(s) at 25°C if
[Al3+] = 0.44 M and E° = –1.66 V?
98. In order to determine the identity of a particular lanthanide metal (M), a voltaic cell is
constructed at 25°C with the anode consisting of the lanthanide metal as the electrode
immersed in a solution of 0.0873 M MCl3, and the cathode consisting of a copper electrode
immersed in a 1.00 M Cu(NO3)2 solution. The two half-reactions are as follows:
M(s) M3+(aq) + 3e–
Cu2+(aq) + 2e– Cu(s)
The potential measured across the cell is 2.68 V. What is the identity of the metal?