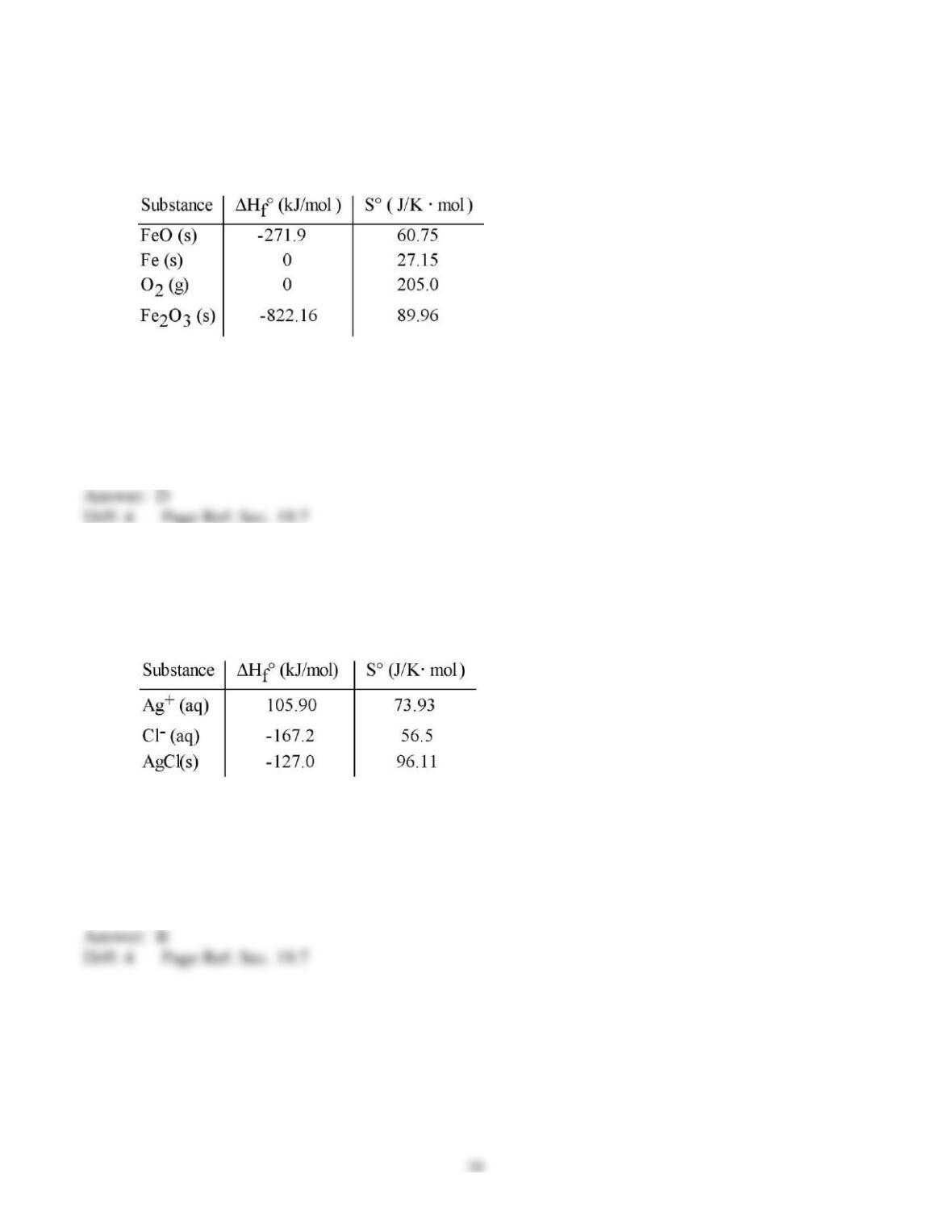
3) Calculate ΔG° (in kJ/mol) for the following reaction at 1 atm and 25°C:
C2H6 (g) + O2 (g) → CO2 (g) + H2O (l) (unbalanced)
ΔHf° C2H6 (g) = -84.7 kJ/mol; S∘C2H6 (g) = 229.5 J/K ∙ mol;
ΔHf° CO2 (g) = -393.5 kJ/mol; S∘CO2 (g) = 213.6 J/K ∙ mol;
ΔHf° H2O (l) = -285.8 kJ/mol; S∘H2O (l) = 69.9 J/K ∙ mol;
S∘O2 (g) = 205.0 J/K ∙ mol
4) Find the temperature (in K) above which a reaction with a ΔH of 123.0 kJ/mol and a ΔS of 90.00 J/K∙
mol becomes spontaneous.
5) Find the temperature (in K) above which a reaction with a ΔH of 53.00 kJ/mol and a ΔS of 100.0 J/K∙
mol becomes spontaneous.
6) Calculate ΔG° for the autoionization of water at 25°C. Kw = 1.0 × 10-14
19.5 True/False Questions
1) The melting of a substance at its melting point is an isothermal process.
2) The vaporization of a substance at its boiling point is an isothermal process
3) The quantity of energy gained by a system equals the quantity of energy gained by its surroundings.
4) The entropy of a pure crystalline substance at 0°C is zero.
5) The more negative ΔG° is for a given reaction, the larger the value of the corresponding equilibrium
constant, K.