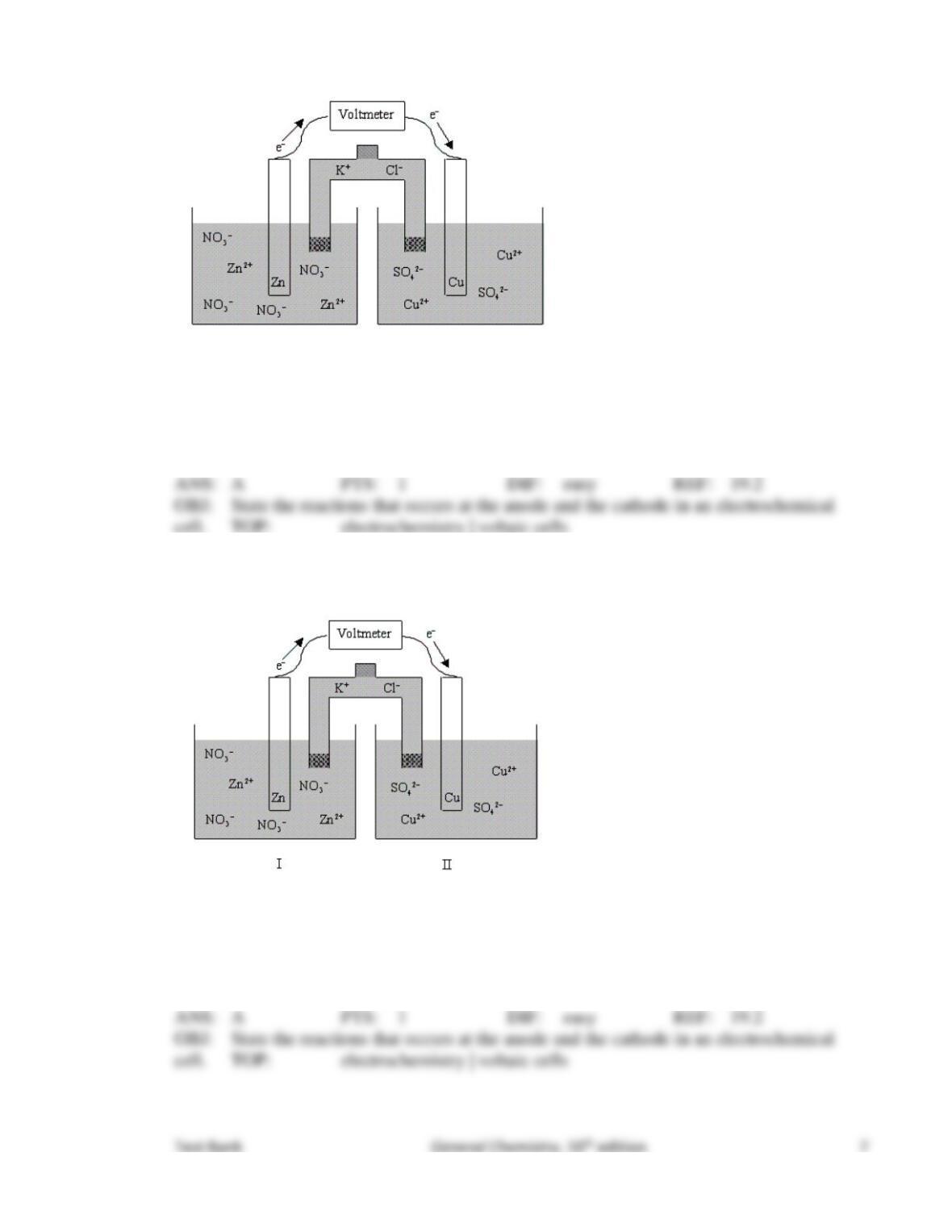
31. Consider the following standard electrode potentials:
Ag+(aq) + e– Ag(s); E° = 0.80 V
Mn2+(aq) + 2e– Mn(s); E° = –1.18 V
Which of the following statements is false concerning the electrochemical cell given below?
Mn(s) | Mn2+(aq) || Ag+(aq) | Ag(s)
The anode half-cell reaction is Mn(s) → Mn2+(aq) + 2e–.
The reducing agent is Ag(s).
Under standard-state conditions, the cell potential is 1.98 V.
The cell potential decreases with time.
The oxidizing agent is Ag+(aq).
32. For a galvanic cell using Fe | Fe2+(1.0 M) and Pb | Pb2+(1.0 M) half-cells, which of the
following statements is correct?
Fe2+(aq) + 2e– → Fe(s); E° = –0.41 V
Pb2+(aq) + 2e– → Pb(s); E° = –0.13 V
The iron electrode is the cathode.
When the cell has completely discharged, the concentration of Pb2+ is zero.
The mass of the iron electrode increases during discharge.
The concentration of Pb2+ decreases during discharge.
Electrons leave the lead electrode to pass through the external circuit during
discharge.
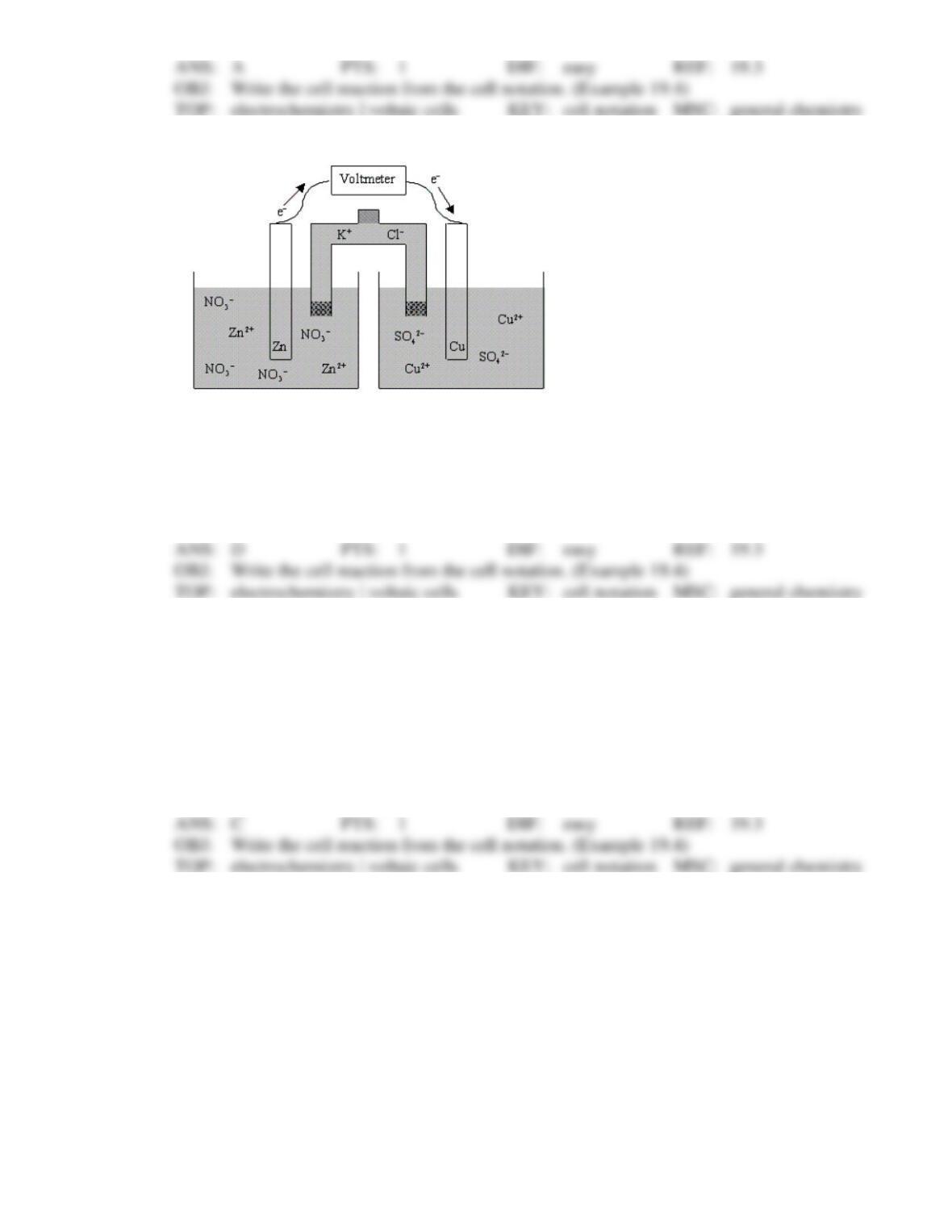
33. What is the cell reaction for the following electrochemical cell?
Ni | Ni2+(aq) || Y3+(aq) | Y
3Ni(s) + 2Y3+(aq) → 2Y(s) + 3Ni2+(aq)
2Y(s) + 3Ni2+(aq) → 3Ni(s) + 2Y3+(aq)
Ni(s) + Ni2+(aq) → Y(s) + Y3+(aq)
Ni(s) + Y3+(aq) → Y(s) + Ni2+(aq)
Y(s) + Ni2+(aq) → Ni(s) + Y3+(aq)