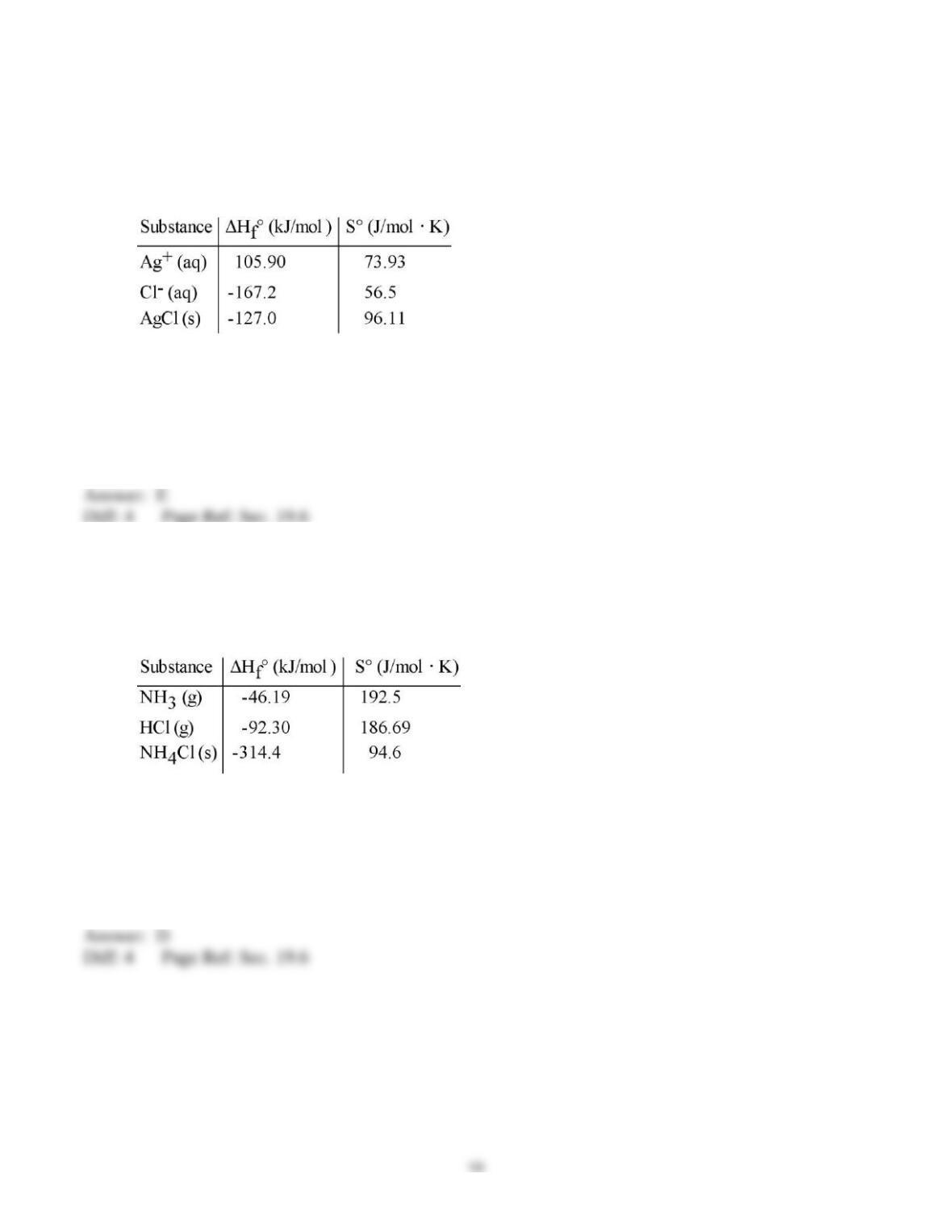
10) The entropy of the universe is __________.
A) constant
B) continually decreasing
C) continually increasing
D) zero
E) the same as the energy, E
11) The second law of thermodynamics states that __________.
A) ΔE = q + w
B)
( ) ( )
o o o
rxn f f
ΔH = nΔH products mΔH reactants−
C) for any spontaneous process, the entropy of the universe increases
D) the entropy of a pure crystalline substance is zero at absolute zero
E) ΔS = qrev/T at constant temperature
12) Which of the following statements is false?
A) The change in entropy in a system depends on the initial and final states of the system and the path
taken from one state to the other.
B) Any irreversible process results in an overall increase in entropy.
C) The total entropy of the universe increases in any spontaneous process.
D) Entropy increases with the number of microstates of the system.
13) Which one of the following processes produces a decrease in the entropy of the system?
A) boiling water to form steam
B) dissolution of solid KCl in water
C) mixing of two gases into one container
D) freezing water to form ice
E) melting ice to form water
14) Which one of the following processes produces a decrease of the entropy of the system?
A) dissolving sodium chloride in water
B) sublimation of naphthalene
C) dissolving oxygen in water
D) boiling of alcohol
E) explosion of nitroglycerine