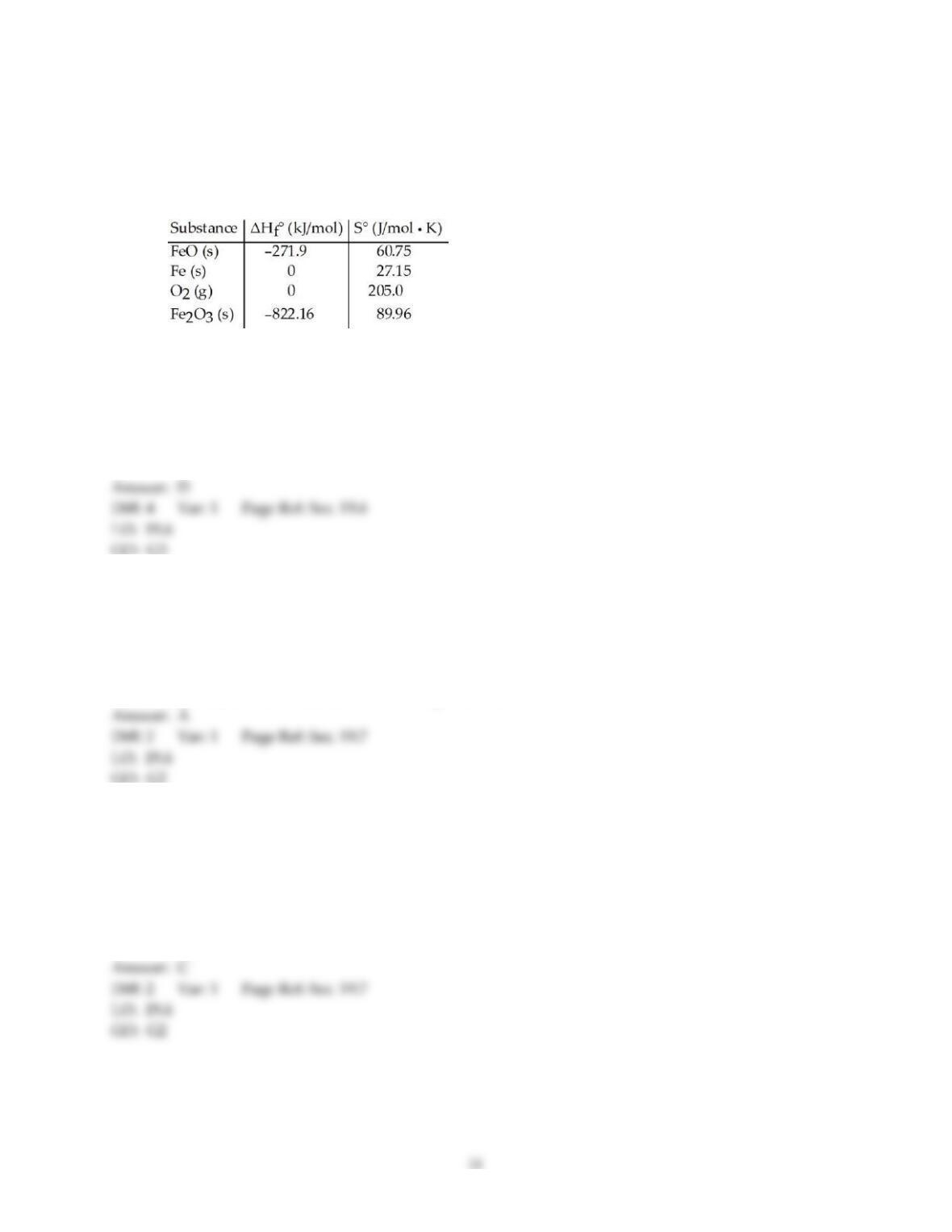
5
16) The entropy change accompanying any process is given by the equation:
A) ΔS = k lnWfinal
B) ΔS = k Wfinal - k Winitial
C) ΔS = k ln(Wfinal / Winitial)
D) ΔS = k final - k initial
E) ΔS = Wfinal - Winitial
17) ΔS is positive for the reaction ________.
A) 2H2 (g) + O2 (g) → 2H2O (g)
B) 2NO2 (g) → N2O4 (g)
C) CO2 (g) → CO2 (s)
D) BaF2 (s) → Ba2+ (aq) + 2F- (aq)
E) 2Hg (l) + O2 (g) → 2HgO (s)
18) ΔS is positive for the reaction ________.
A) 2NO (g) + O2 (g) → 2NO2 (g)
B) 2N2 (g) + 3H2 (g) → 2NH3 (g)
C) C3H8 (g) + 5 O2 (g) → 3CO2 (g) + 4 H2O (g)
D) Mg (s) + Cl2 (g) → MgCl2 (s)
E) C2H4 (g) + H2 (g) → C2H6 (g)
19) ΔS is positive for the reaction ________.
A) CaO (s) + CO2 (g) → CaCO3 (s)
B) N2 (g) + 3H2 (g) → 2NH3 (g)
C) 2SO3 (g) → 2SO2 (g) + O2 (g)
D) Ag+ (aq) + Cl- (aq) → AgCl (s)
E) H2O (l) → H2O (s)
20) ΔS is positive for the reaction ________.
A) 2 Ca (s) + O2 (g) → 2 CaO (s)