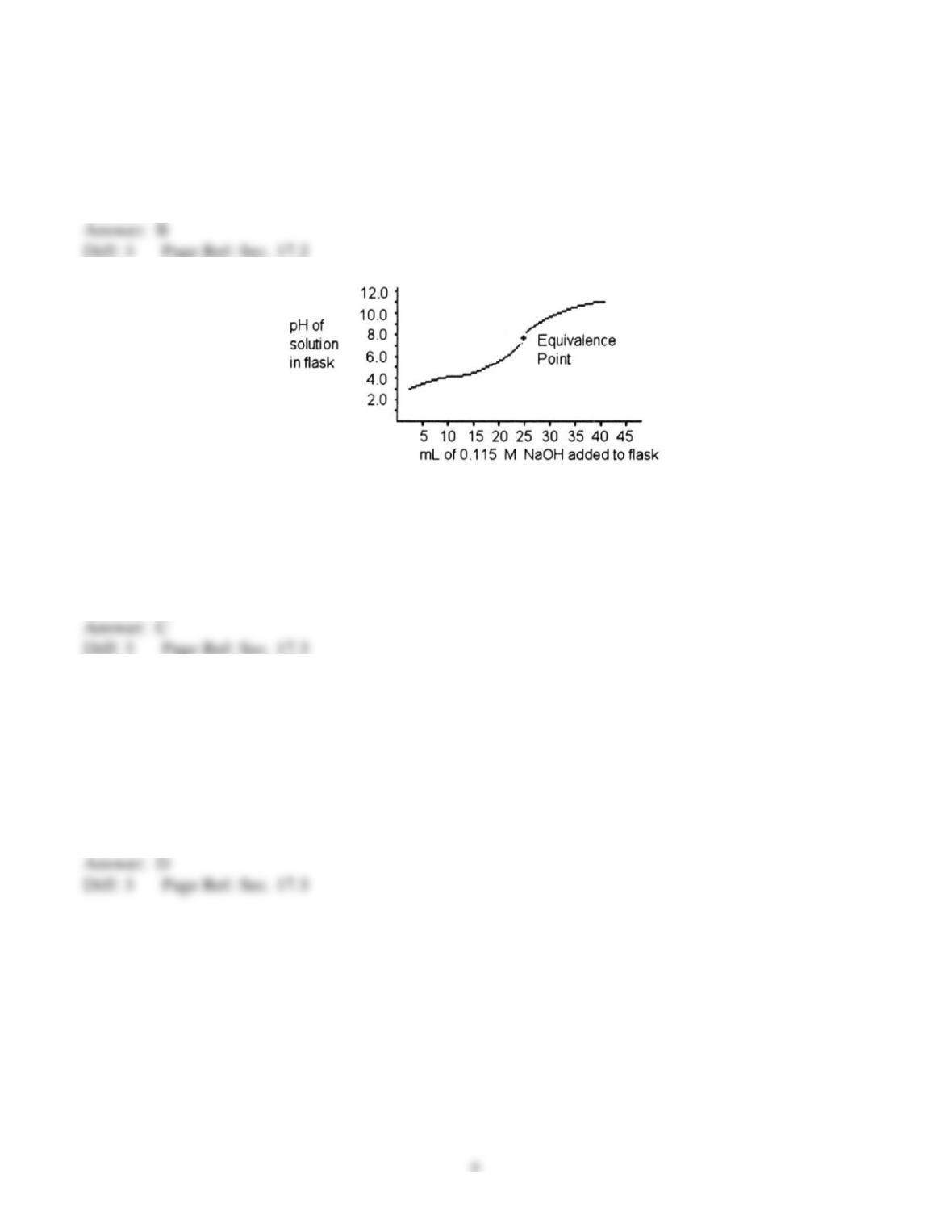
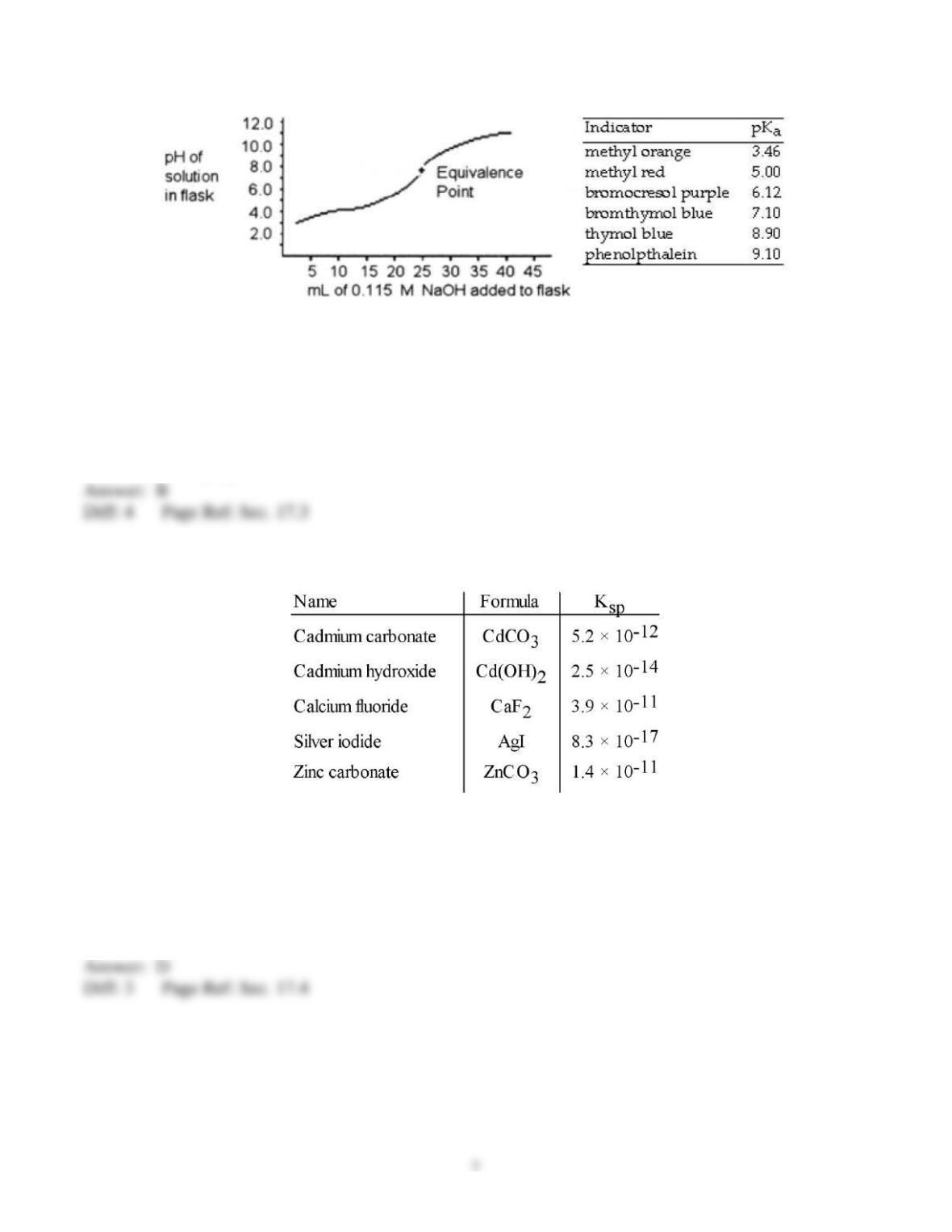
9) Of the following solutions, which has the greatest buffering capacity?
A) 0.821 M HF and 0.217 M NaF
B) 0.821 M HF and 0.909 M NaF
C) 0.100 M HF and 0.217 M NaF
D) 0.121 M HF and 0.667 M NaF
E) They are all buffer solutions and would all have the same capacity.
10) Of the following solutions, which has the greatest buffering capacity?
A) 0.521 M HC2H3O2 and 0.217 M NaC2H3O2
B) 0.821 M HC2H3O2 and 0.713 M NaC2H3O2
C) 0.365M HC2H3O2 and 0.497 M NaC2H3O2
D) 0.121 M HC2H3O2 and 0.116 M NaC2H3O2
11) Of the following solutions, which has the greatest buffering capacity?
A) 0.543 M NH3 and 0.555 M NH4Cl
B) 0.087 M NH3 and 0.088 M NH4Cl
C) 0.234 M NH3 and 0.100 M NH4Cl
D) 0.100 M NH3 and 0.455 M NH4Cl
E) They are all buffer solutions and would all have the same capacity.
12) The addition of hydrofluoric acid and __________ to water produces a buffer solution.
A) HCl
B) NaNO3
C) NaF
D) NaCl
E) NaBr
13) The addition of hydrofluoric acid and __________ to water produces a buffer solution.
A) HCl
B) NaNO3
C) NaCl
D) NaOH
E) NaBr