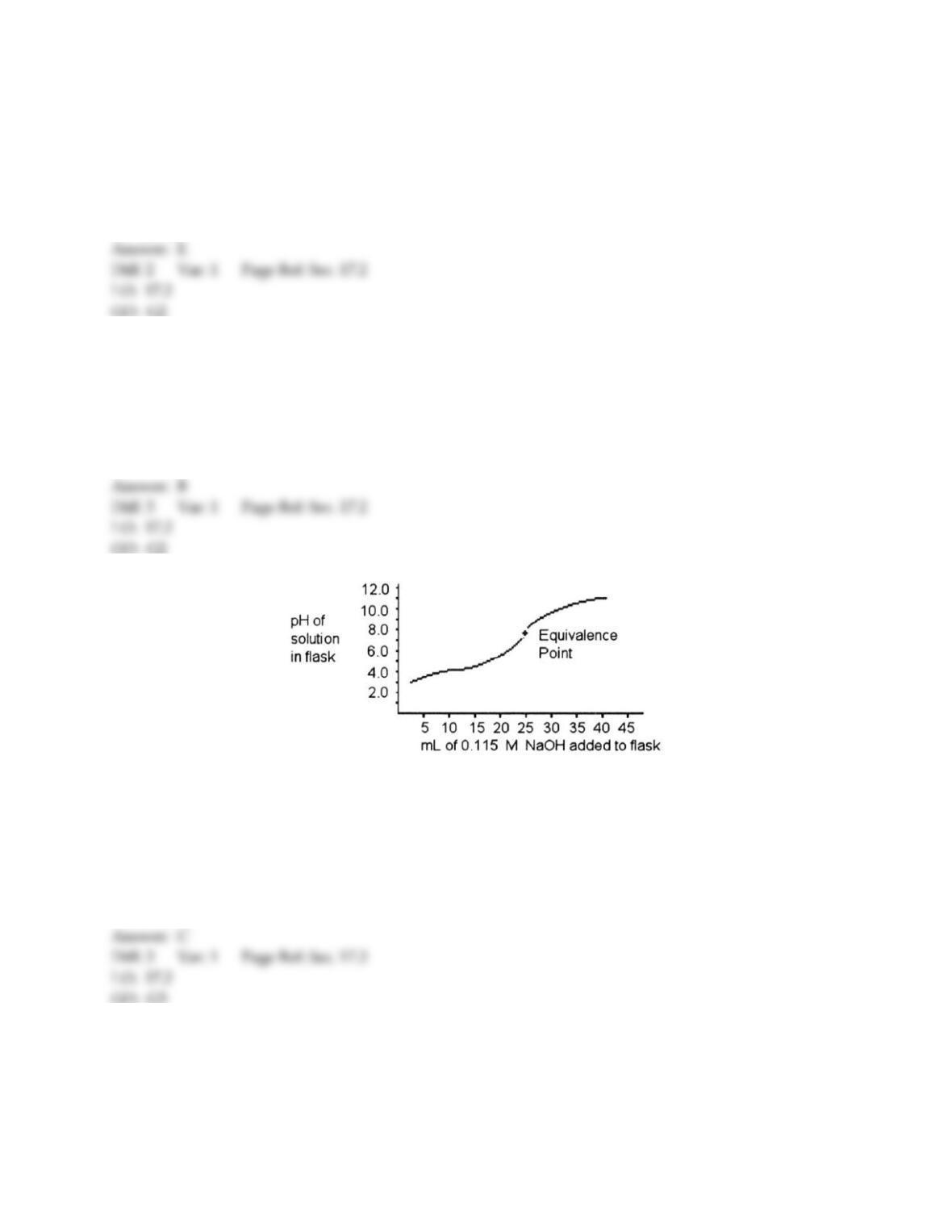
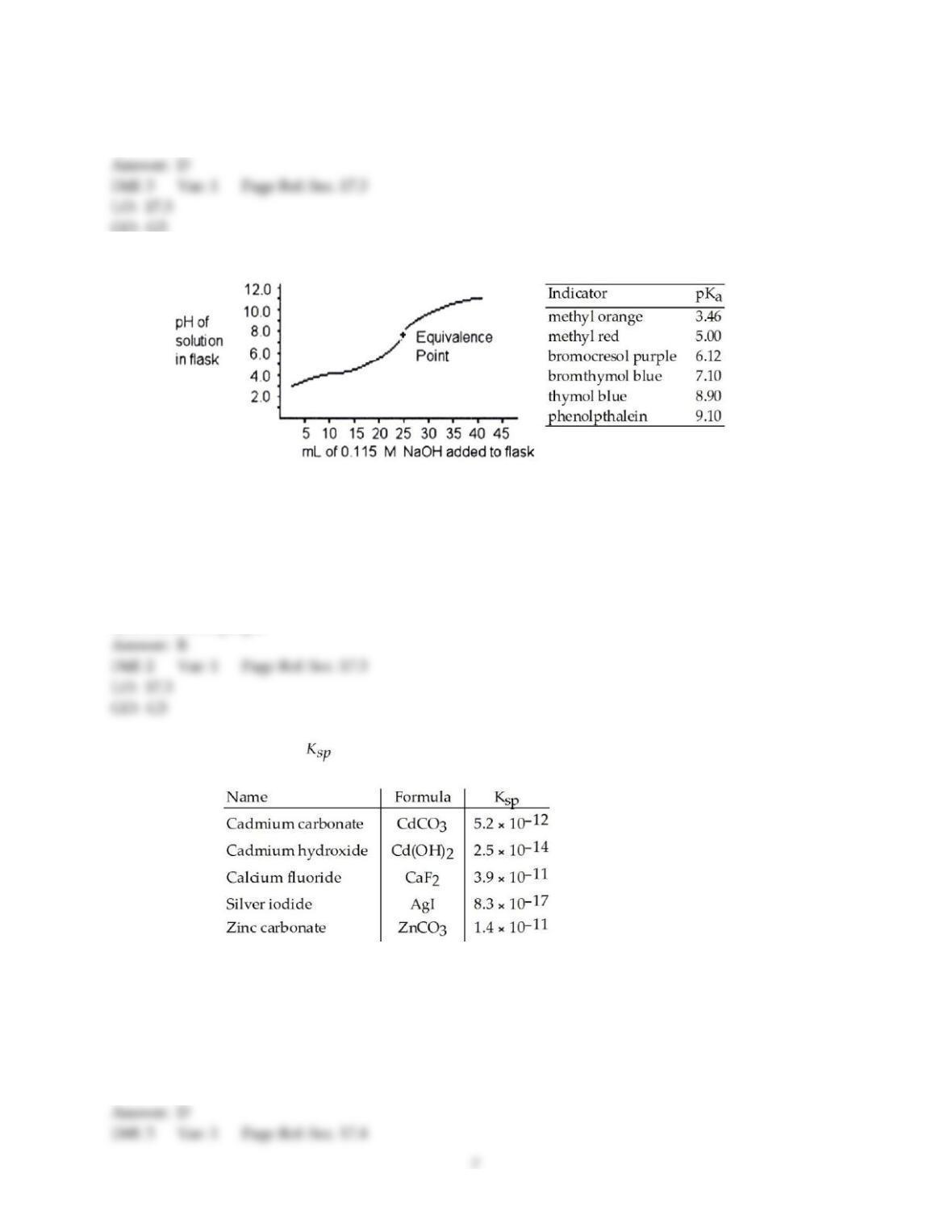
9
A) Ca(NO3)2
B) CaF2
C) CaCl2
D) CaBr2
E) CaI2
32) Which below best describe(s) the behavior of an amphoteric hydroxide in water?
A) With conc. aq. NaOH, its suspension dissolves.
B) With conc. aq. HCl, its suspension dissolves.
C) With conc. aq. NaOH, its clear solution forms a precipitate.
D) With conc. aq. HCl, its clear solution forms a precipitate.
E) With both conc. aq. NaOH and conc. aq. HCl, its suspension dissolves.
33) Why does fluoride treatment render teeth more resistant to decay?
A) Fluoride kills the bacteria in the mouth that make the acids that decay teeth.
B) Fluoride stimulates production of tooth enamel to replace that lost to decay.
C) Fluoride reduces saliva production, keeping teeth drier and thus reducing decay.
D) Fluoride converts hydroxyapatite to fluoroapatite that is less reactive with acids.
E) Fluoride dissolves plaque, reducing its decaying contact with teeth.
34) A result of the common-ion effect is ________.
A) that some ions, such as Na+ (aq), frequently appear in solutions but do not participate in solubility
equilibria
B) that common ions, such as Na+ (aq), don't affect equilibrium constants
C) that the selective precipitation of a metal ion, such as Ag+, is promoted by the addition of an
appropriate counterion (X-) that produces a compound (AgX) with a very low solubility
D) that ions such as K+ and Na+ are common ions, so that their values in equilibrium constant
expressions are always 1.00
E) that common ions precipitate all counter-ions
17.2 Bimodal Questions
1) The Ka of benzoic acid is 6.30 × 10-5. The pH of a buffer prepared by combining 50.0 mL of 1.00 M
potassium benzoate and 50.0 mL of 1.00 M benzoic acid is ________.