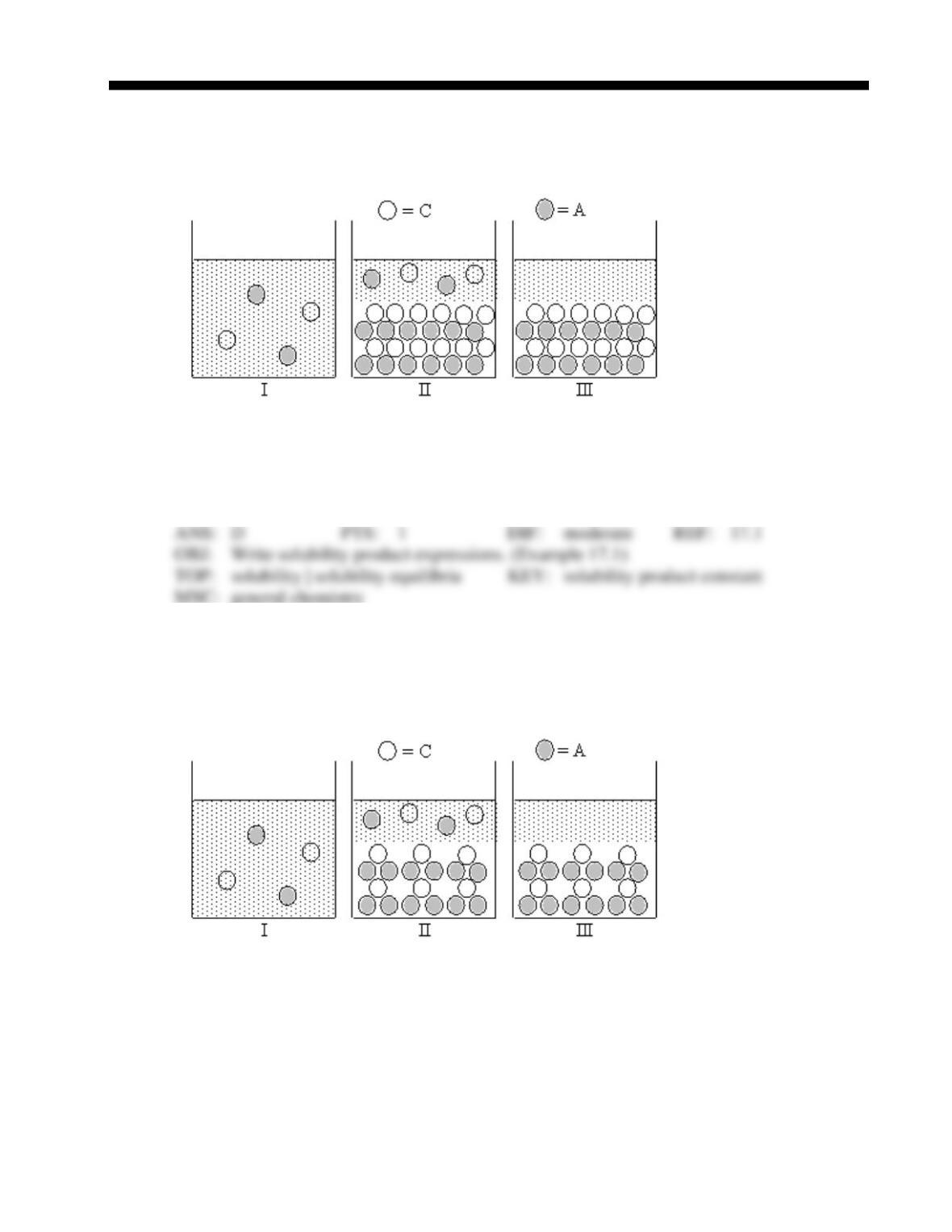
45. The insoluble salts AV, B2W, C2X3, DY2, and EZ3, which were formed from the metal ions
A+, B+, C3+, D2+, and E3+ and the nonmetals V1–, W2–, X2–, Y1–, and Z1–, all have the same
Ksp value. Which salt has the highest molar solubility?
46. In which of the following solutions would silver(I) phosphate, Ag3PO4, be least soluble?
47. In which of these solutions would silver(I) carbonate have the lowest molar solubility? For
silver(I) carbonate, Ksp = 8.5 10–12.