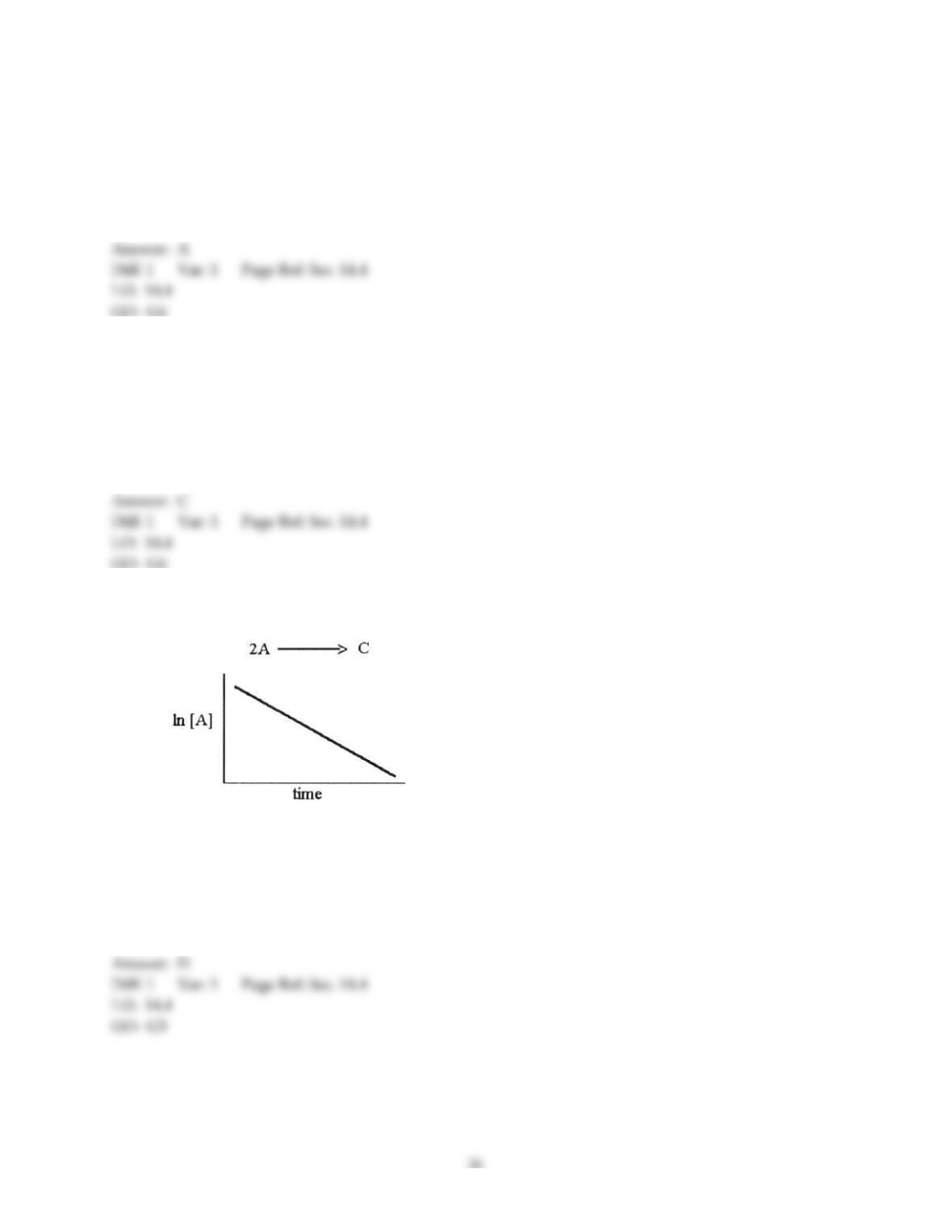
29
4) The rate law for a reaction is
rate = k[A][B]
Which one of the following statements is false?
A) The reaction is first order overall.
B) The reaction is first order in A.
C) The reaction is first order in [B].
D) k is the reaction rate constant
E) If [A] is doubled, the reaction rate will increase by a factor of 2.
5) Nitrogen dioxide decomposes to nitric oxide and oxygen via the reaction:
2NO2 → 2NO + O2
In a particular experiment at 300 °C, [NO2] drops from 0.0143 to 0.00701 M in 261 s. The rate of
disappearance of NO2 for this period is ________ M/s.
A) 2.79 × 10-5
B) -8.16 × 10-5
C) 5.59 × 10-5
D) 1.40 × 10-5
E) 35,800
6) At elevated temperatures, dinitrogen pentoxide decomposes to nitrogen dioxide and oxygen:
2N2O5(g) → 4NO2 (g) + O2 (g)
When the rate of formation of O2 is 2.2 × 10-4 M/s, the rate of decomposition of N2O5 is ________ M/s.
A) 7.2 × 10-3
B) 3.6 × 10-3
C) 1.8 × 10-3
D) 1.4 × 10-2
E) 0.00090
7) The rate of disappearance of HBr in the gas phase reaction