14
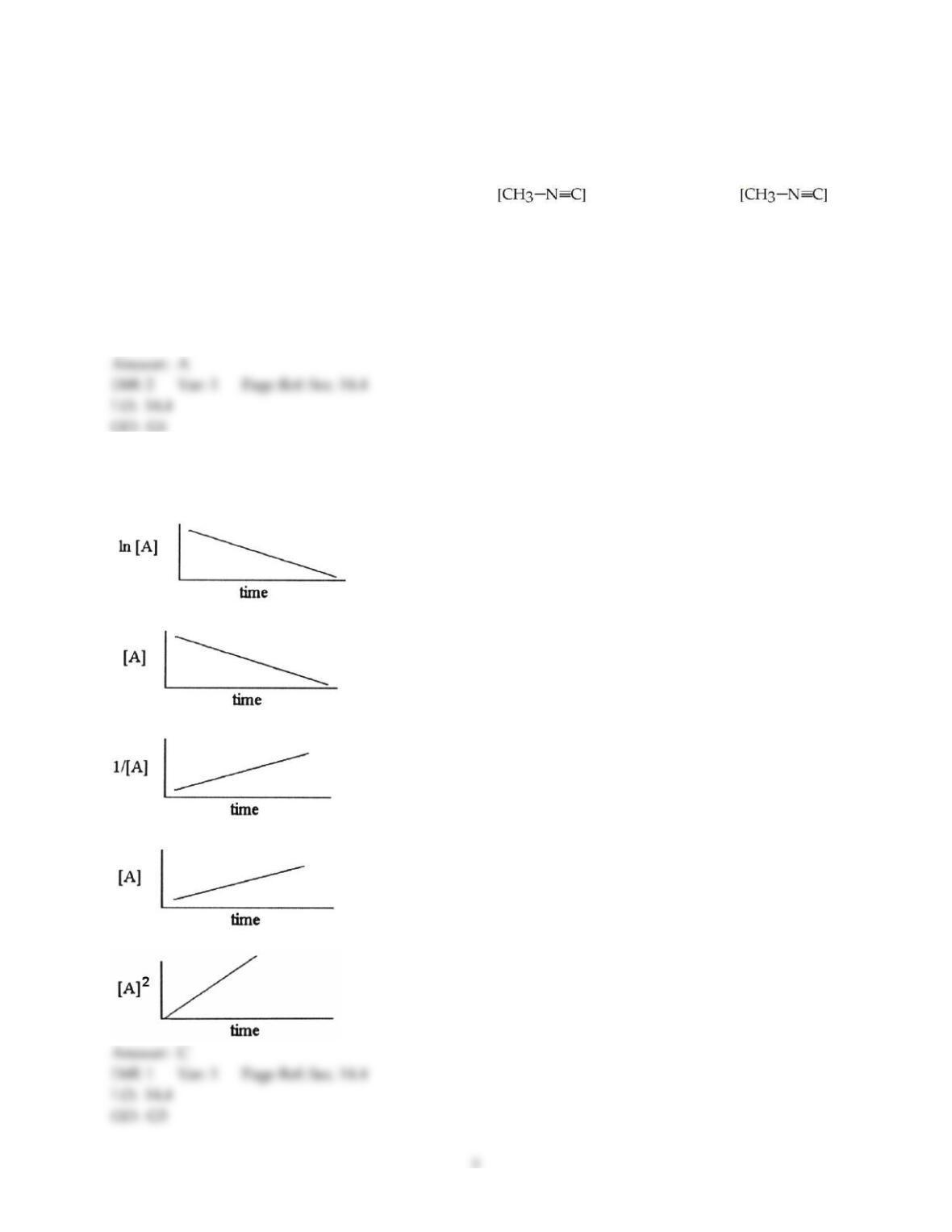
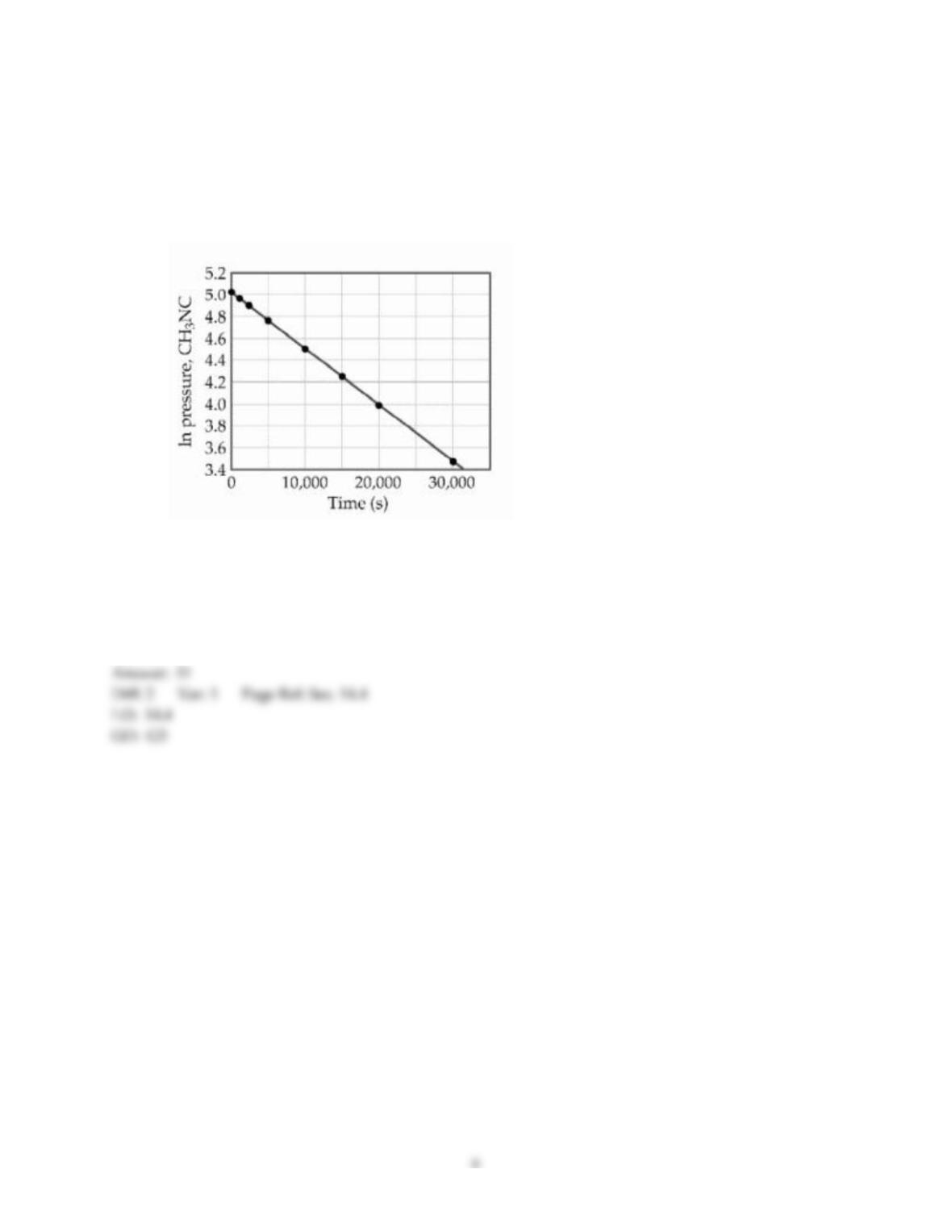
36) The rate law of the overall reaction
A + B → C
is rate = k[A]2. Which of the following will not increase the rate of the reaction?
A) increasing the concentration of reactant A
B) increasing the concentration of reactant B
C) increasing the temperature of the reaction
D) adding a catalyst for the reaction
E) All of these will increase the rate.
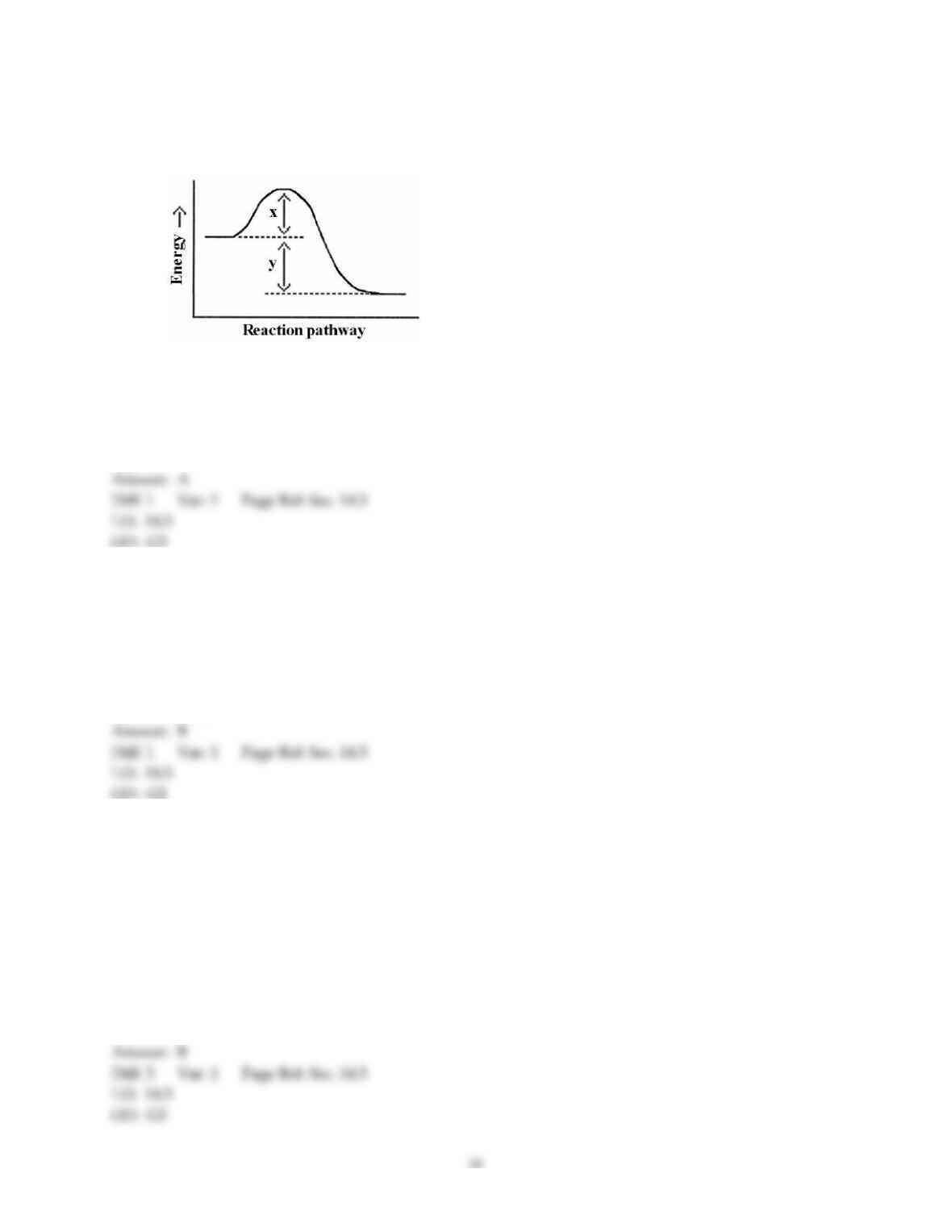
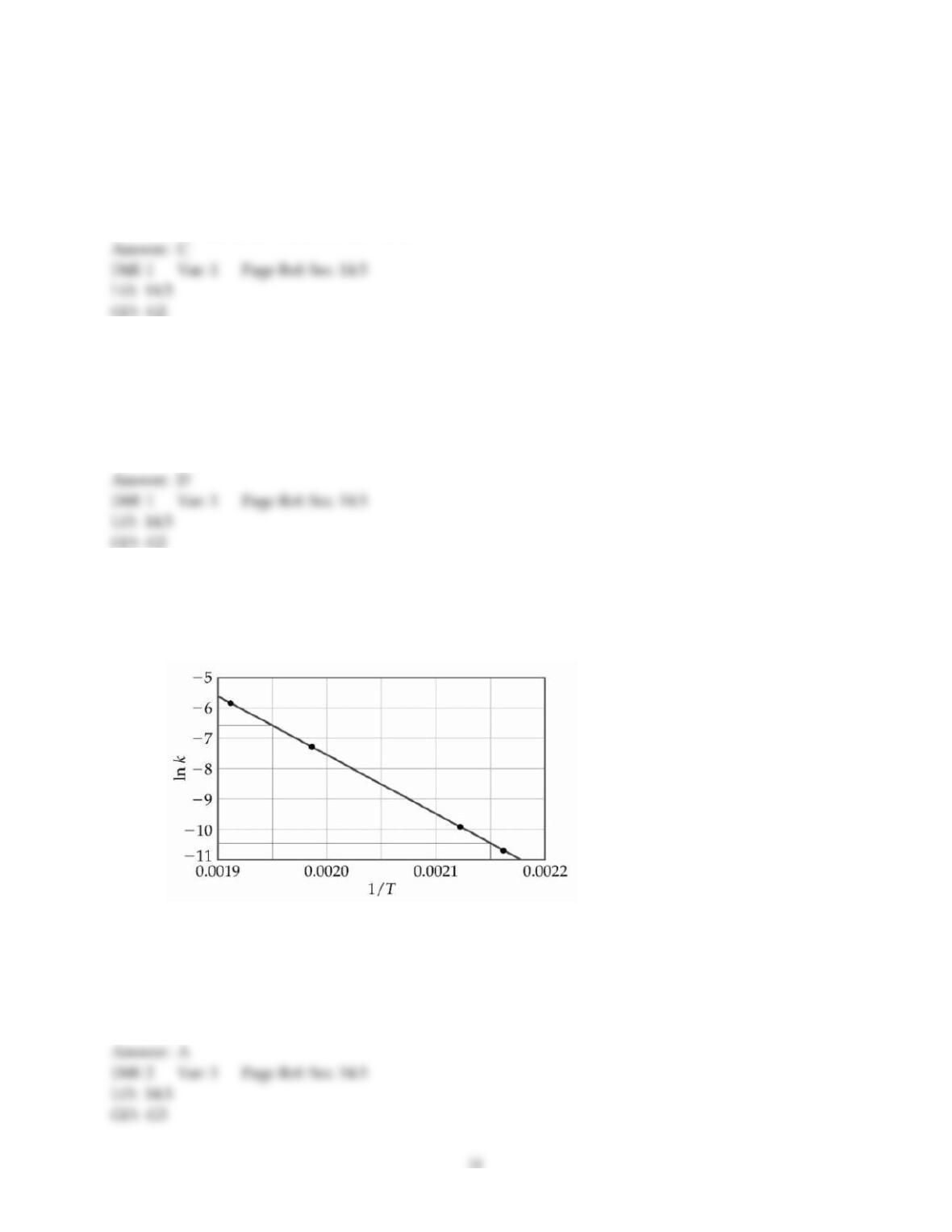
37) A catalyst can increase the rate of a reaction ________.
A) by changing the value of the frequency factor (A)
B) by increasing the overall activation energy (Ea) of the reaction
C) by lowering the activation energy of the reverse reaction
D) by providing an alternative pathway with a lower activation energy
E) All of these are ways that a catalyst might act to increase the rate of reaction.
38) The primary source of the specificity of enzymes is ________.
A) their polarity, which matches that of their specific substrate
B) their delocalized electron cloud
C) their bonded transition metal, which is specific to the target substrate
D) their locations within the cell
E) their shape, which relates to the lock-and-key model
39) ________ are used in automotive catalytic converters.
A) Heterogeneous catalysts
B) Homogeneous catalysts
C) Enzymes
D) Noble gases
E) Nonmetal oxides
40) The enzyme nitrogenase converts ________ into ________.