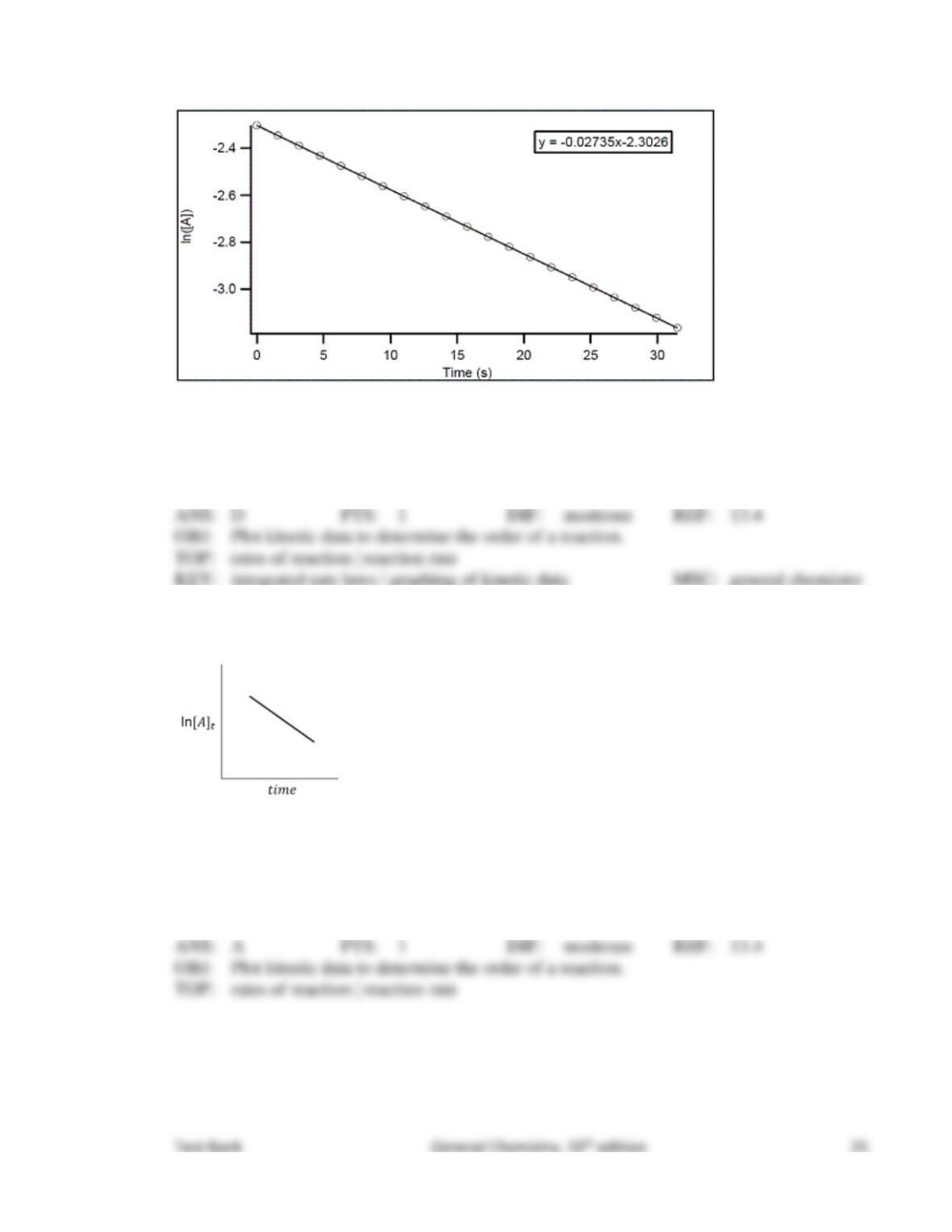
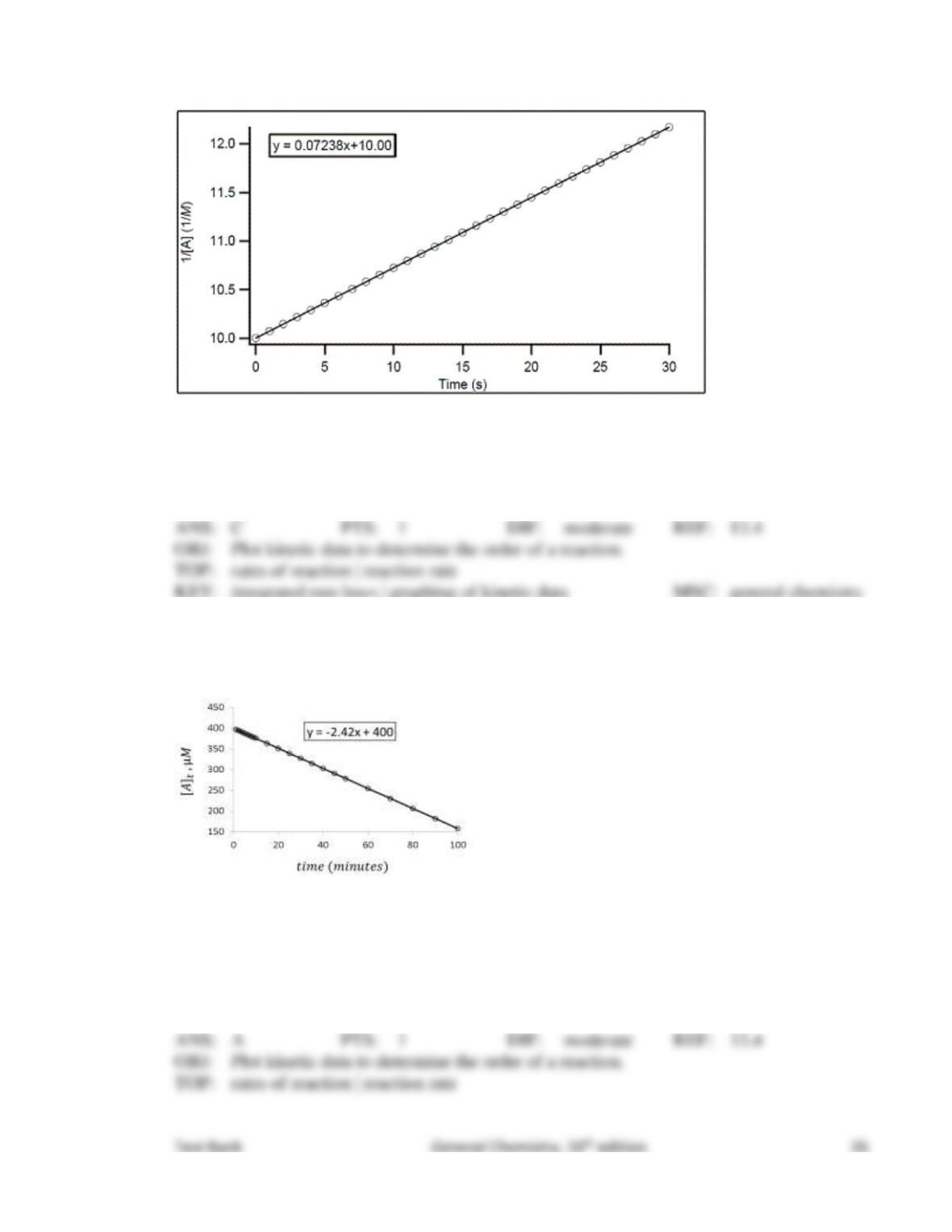
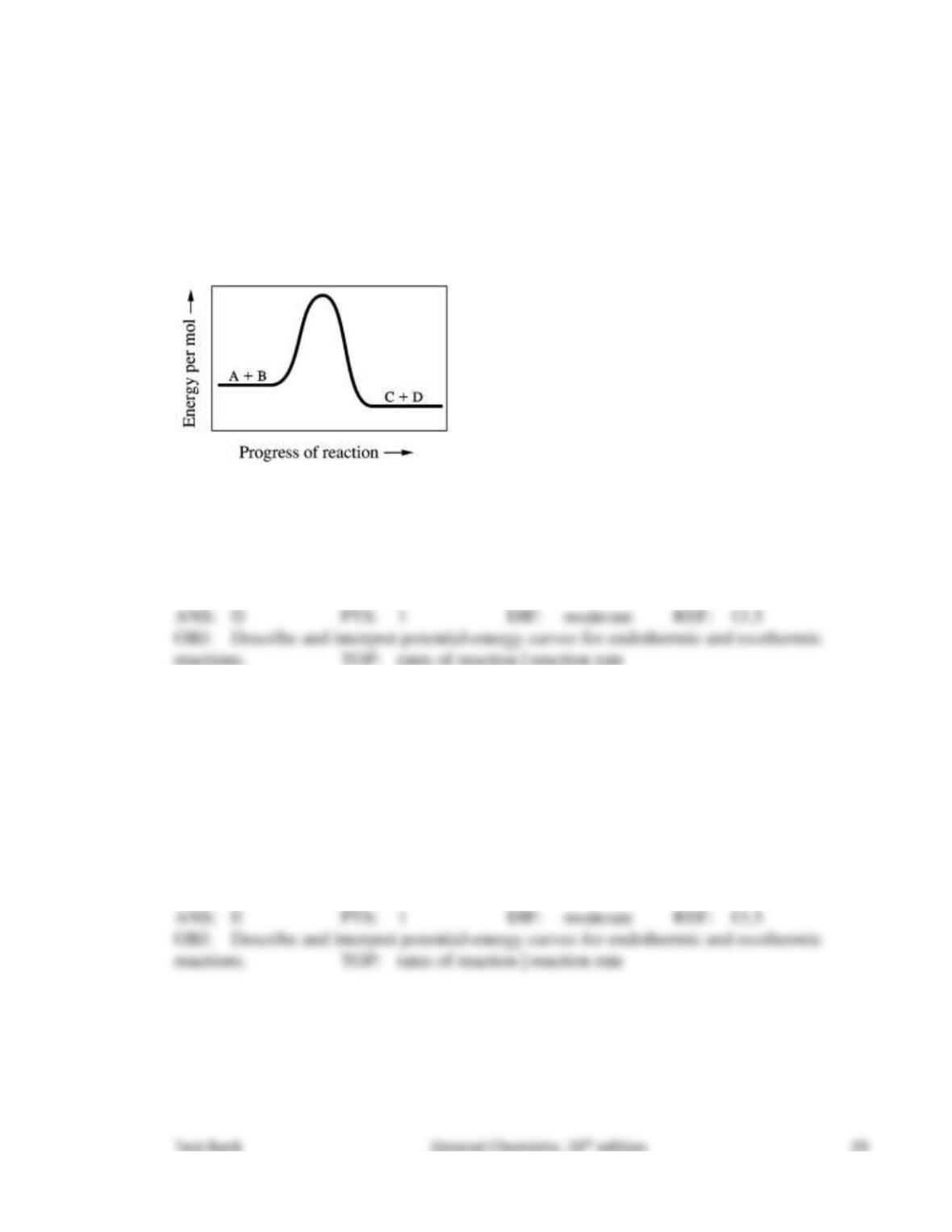
77. What would happen if the kinetic energy of the reactants were not enough to provide the
needed activation energy?
The rate of the reaction would tend to increase.
The reactants would continue to exist in their present form.
The activated complex would be converted into products.
The products would be produced at a lower energy state.
The products would form at an unstable energy state.
78. The main reason for the increase in reaction rate with temperature is that
the fraction of high-energy molecules increases exponentially with temperature.
the activation energy increases rapidly with temperature.
a 10°C temperature rise results in the rate doubling.
there is a dramatic increase in the number of collisions.
79. When the concentrations of the reactants are increased, the rate of the reaction increases.
This is best explained by
an increase in the fraction of molecules that have enough energy to react.
an increase in the rate constant.
an increase in the average potential energy of the molecules.
an increase in the frequency of the molecular collisions.
an increase in the kinetic energy of the molecules.
80. The rates of most chemical reactions are sensitive to a change in the temperature of the
reaction system. The increase in rate as the temperature increases is best explained by
an increase in the collision frequency.
an increase in the number of high-energy molecules.
a decrease in the collision frequency.
an increase in the activation energy.
a decrease in the activation energy.