4. Consider the reaction:
At a certain instant the initial rate of disappearance of the oxygen gas is X. What is the value
of the appearance of water at the same instant?
cannot be determined from the data
5. For the reaction , at a particular instant in time, the rate of the reaction
is 0.0223 M/s. What is the rate of change of A?
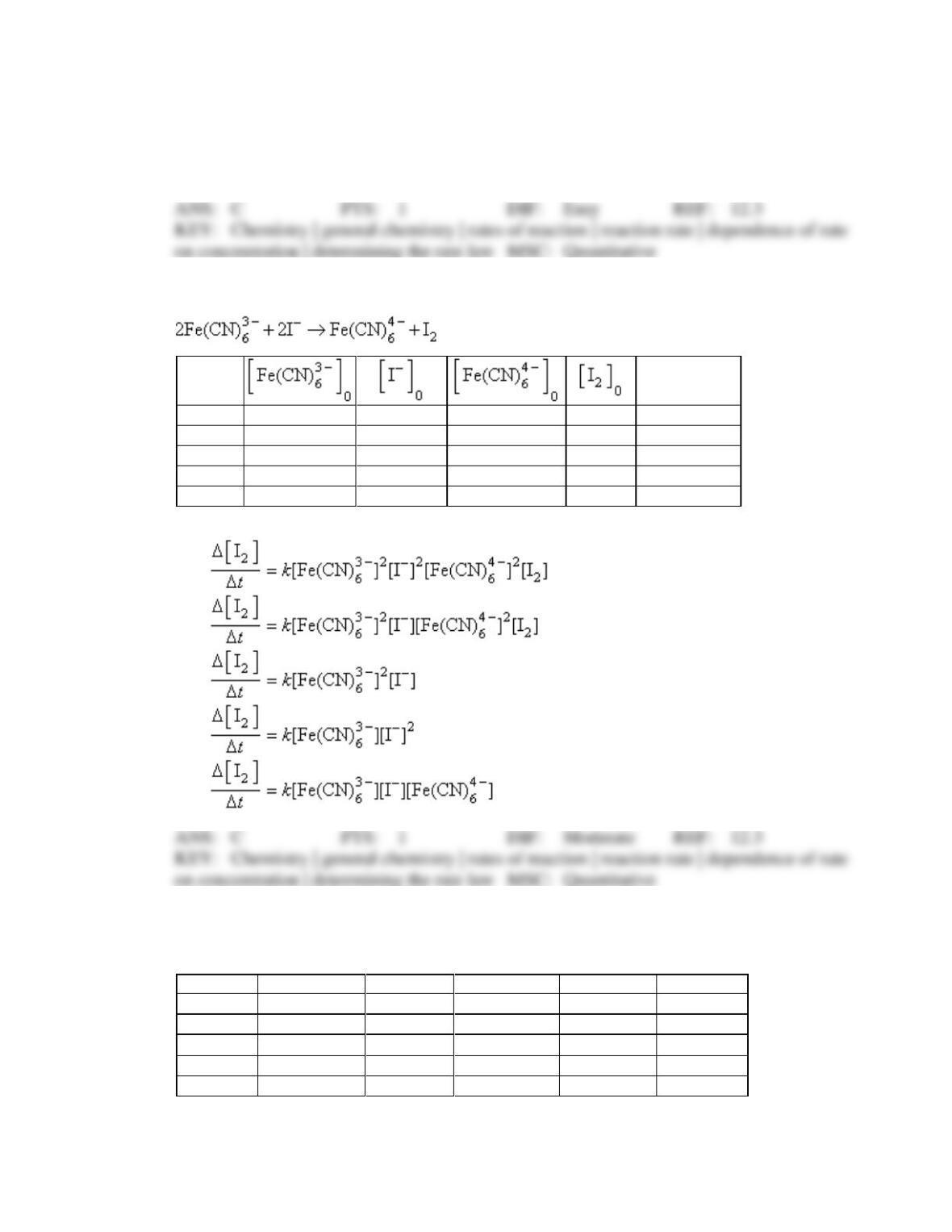
6. Consider the reaction X → Y + Z
Which of the following is a possible rate law?
7. Consider the following rate law:
How are the exponents n and m determined?
by using the balanced chemical equation
by using the subscripts for the chemical formulas
by using the coefficients of the chemical formulas