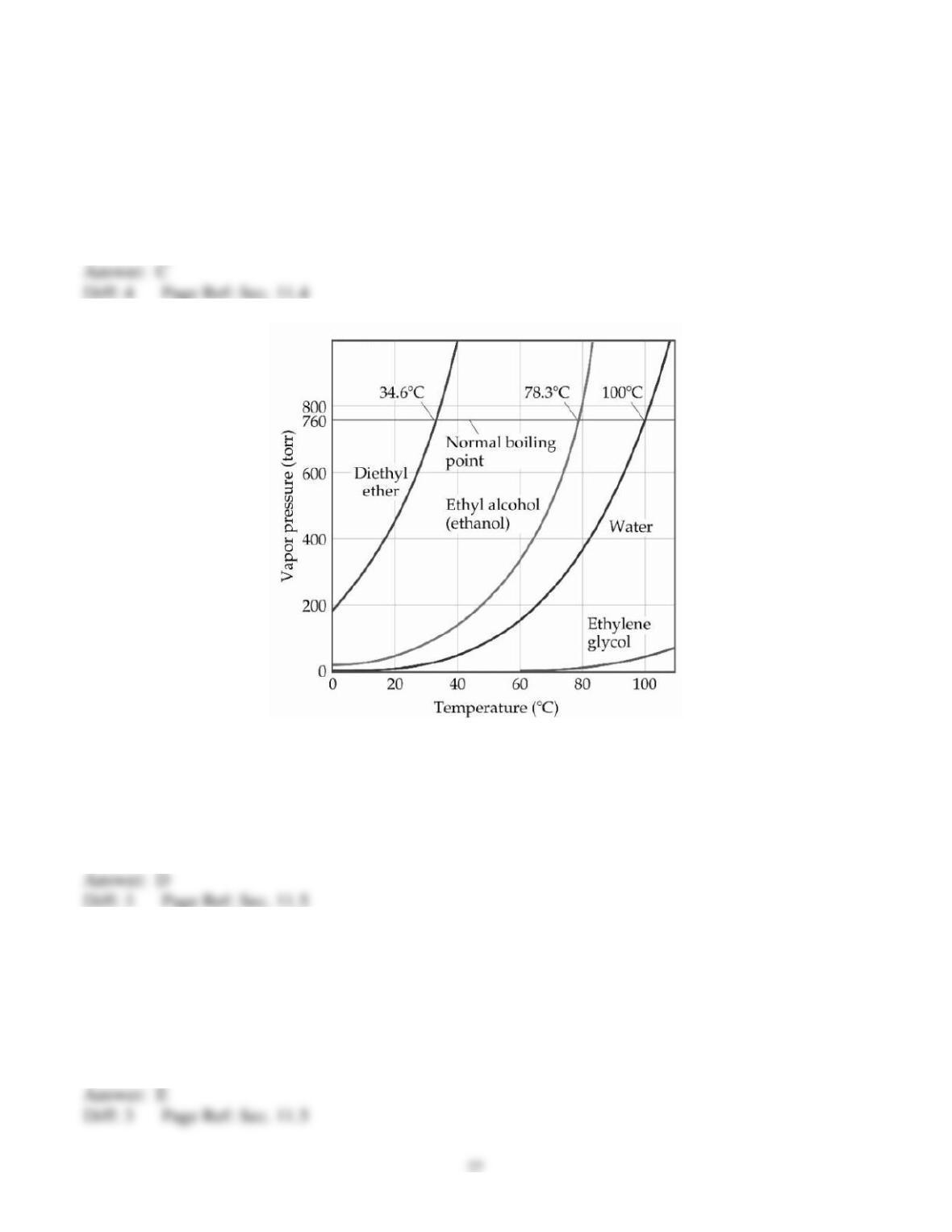
66) Some things take longer to cook at high altitudes than at low altitudes because __________.
A) water boils at a lower temperature at high altitude than at low altitude
B) water boils at a higher temperature at high altitude than at low altitude
C) heat isn't conducted as well in low density air
D) natural gas flames don't burn as hot at high altitudes
E) there is a higher moisture content in the air at high altitude
67) The vapor pressure of a liquid __________.
A) increases linearly with increasing temperature
B) increases nonlinearly with increasing temperature
C) decreases linearly with increasing temperature
D) decreases nonlinearly with increasing temperature
E) is totally unrelated to its molecular structure
68) The slope of a plot of the natural log of the vapor pressure of a substance versus 1/T is __________.
A) ΔHvap
B) -ΔHvap
C)
D)
E)
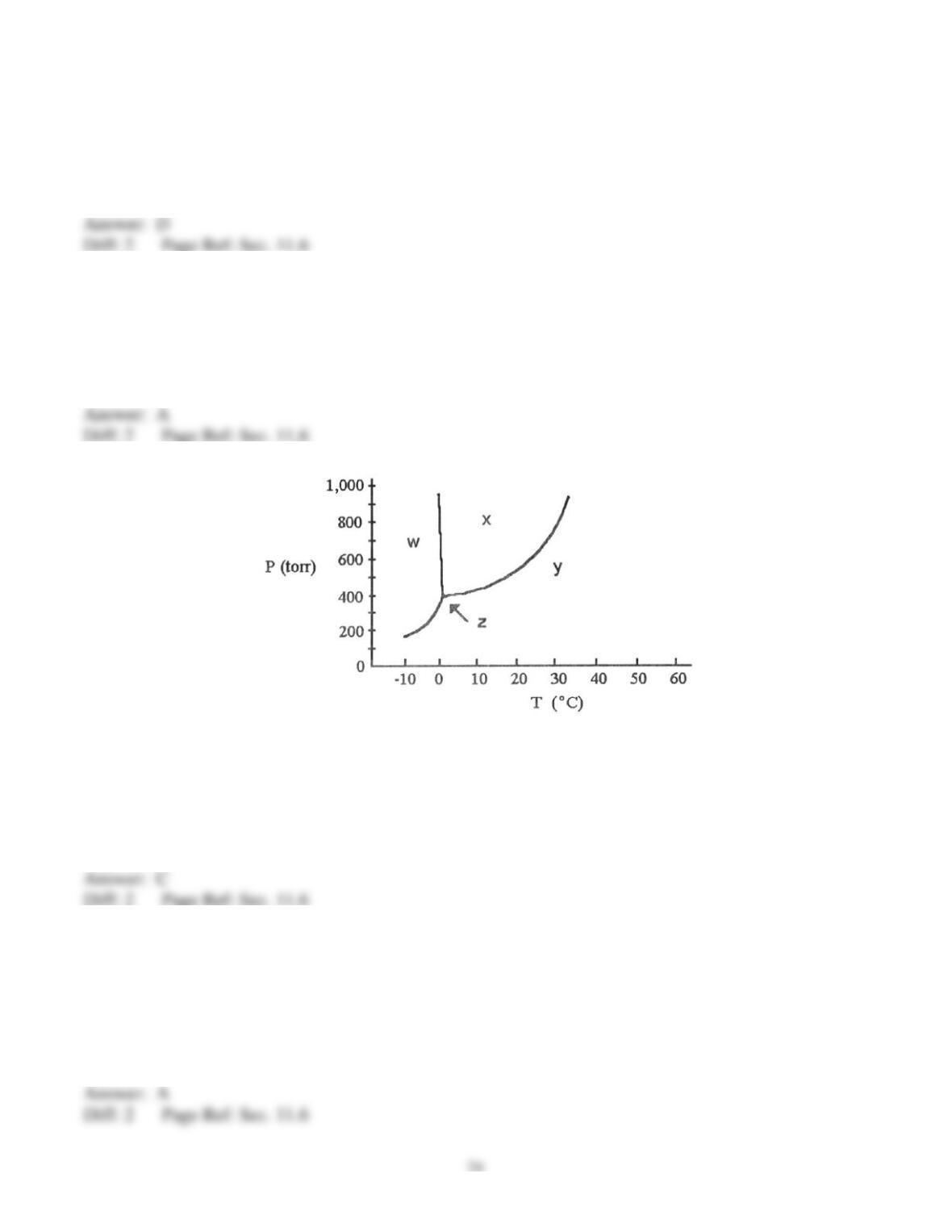
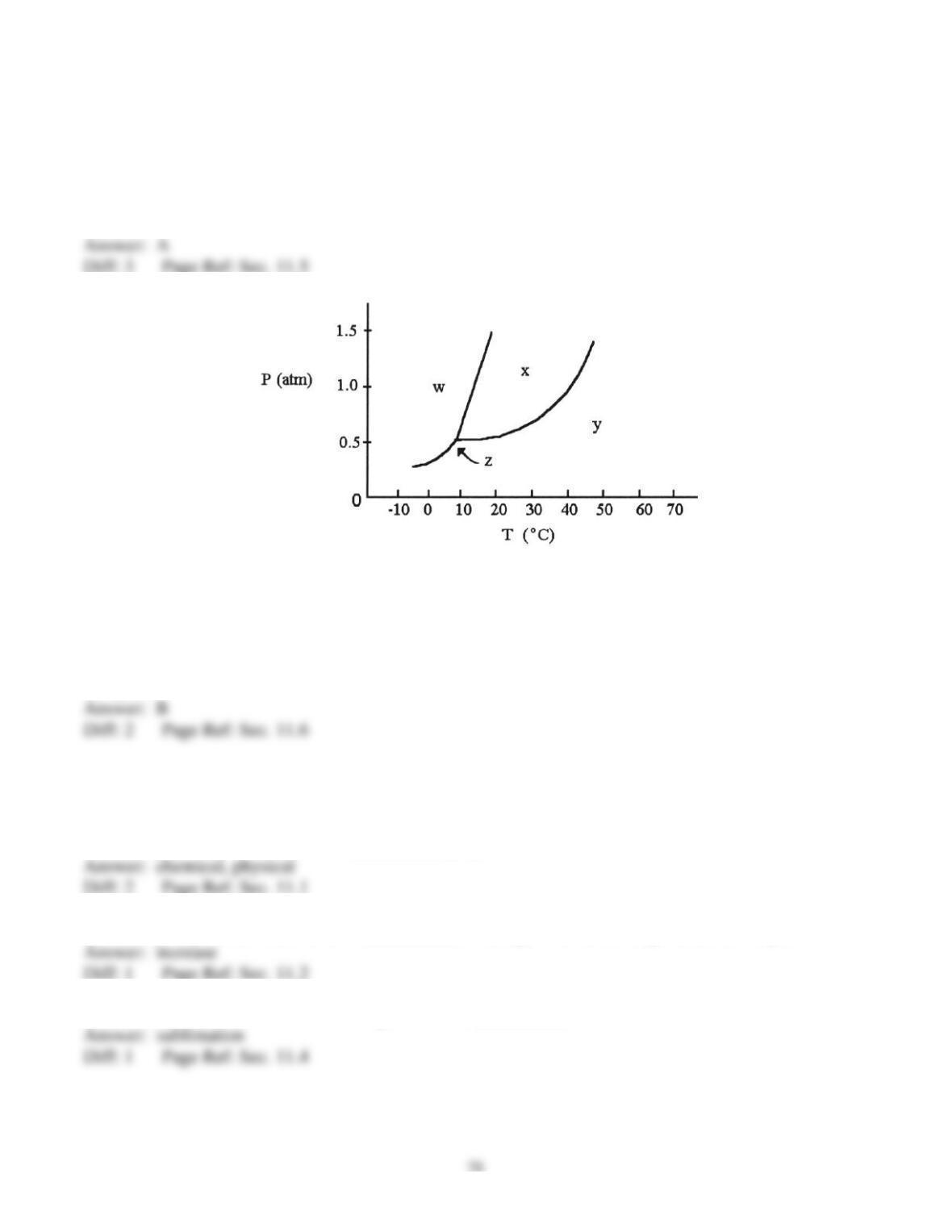
69) On a phase diagram, the critical pressure is __________.
A) the pressure required to melt a solid
B) the pressure below which a substance is a solid at all temperatures
C) the pressure above which a substance is a liquid at all temperatures
D) the pressure at which a liquid changes to a gas
E) the pressure required to liquefy a gas at its critical temperature
70) On a phase diagram, the critical temperature is __________.
A) the temperature below which a gas cannot be liquefied
B) the temperature above which a gas cannot be liquefied
C) the temperature at which all three states are in equilibrium
D) the temperature required to melt a solid
E) the temperature required to cause sublimation of a solid