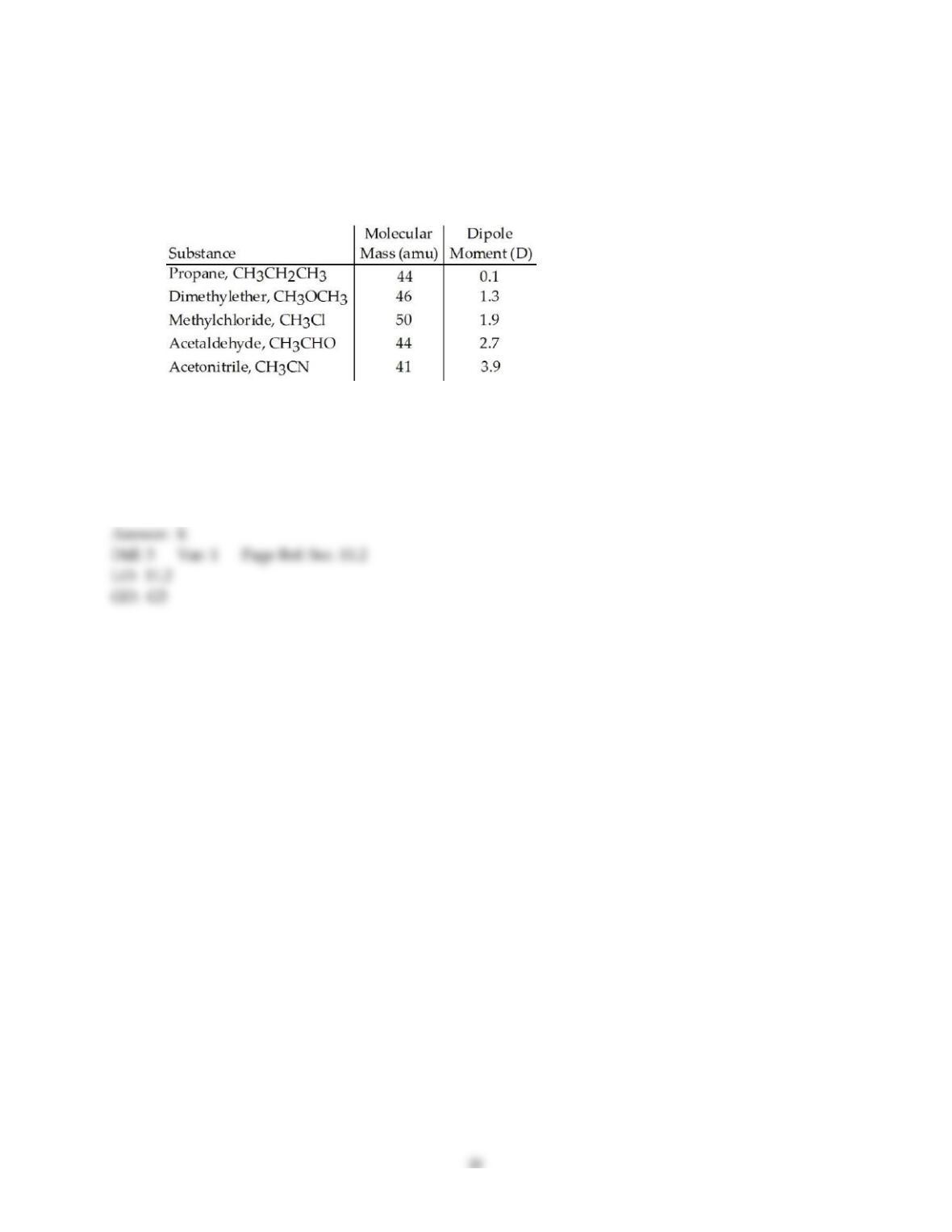
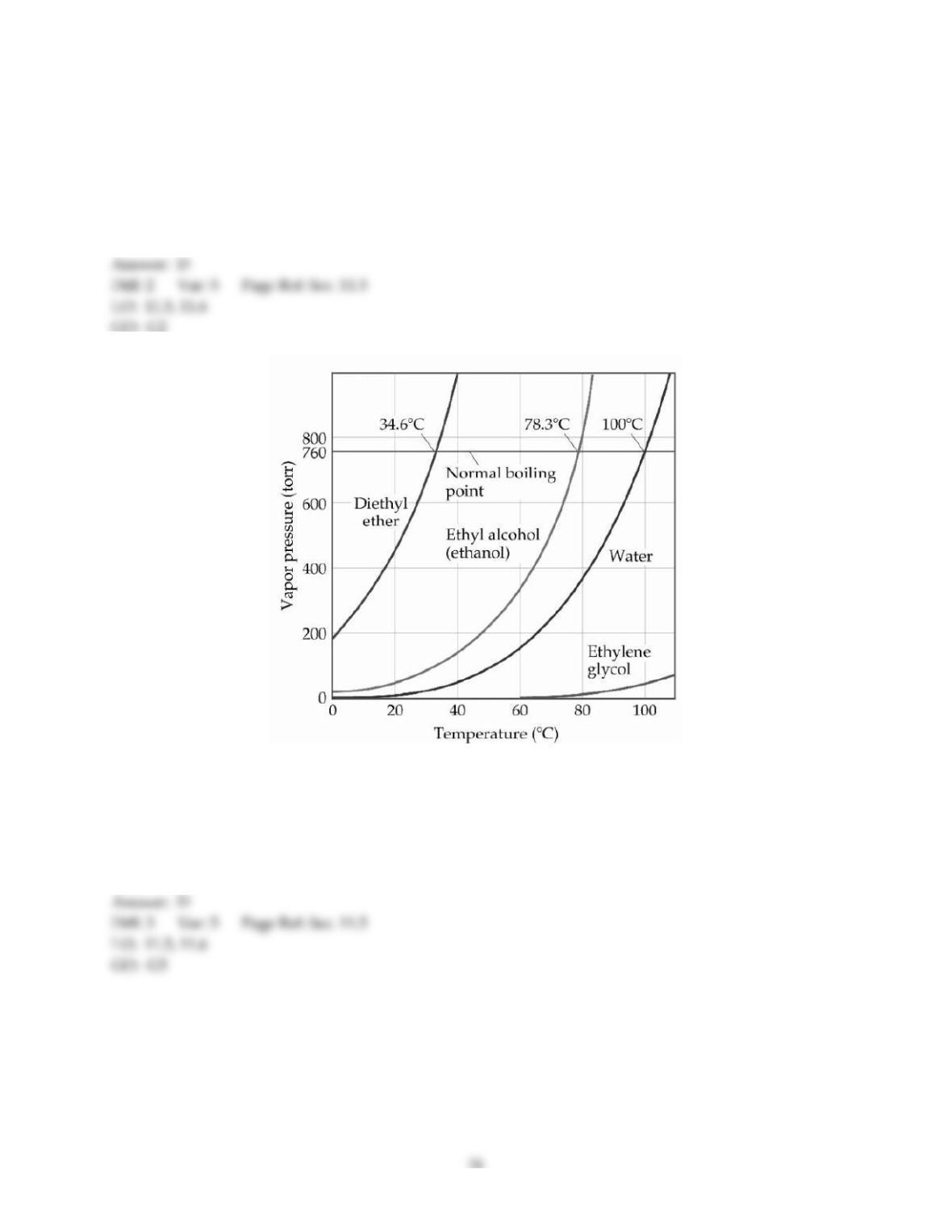
20) The enthalpy change for converting 1.00 mol of ice at -25.0 °C to water at 50.0 °C is ________ kJ. The
specific heats of ice, water, and steam are 2.09 J/g-K, 4.18 J/g-K, and 1.84 J/g-K, respectively. For H2O,
ΔHfus = 6.01 kJ/mol, and ΔHvap = 40.67 kJ/mol.
A) 12.28
B) 6.27
C) 10.71
D) 4709
E) 8.83
21) The enthalpy change for converting 10.0 g of ice at -50.0 °C to water at 50.0 °C is ________ kJ. The
specific heats of ice, water, and steam are 2.09 J/g-K, 4.18 J/g-K, and 1.84 J/g-K, respectively. For H2O,
ΔHfus = 6.01 kJ/mol, and ΔHvap = 40.67 kJ/mol.
A) 12.28
B) 4.38
C) 3138
D) 6.47
E) 9.15
22) The heat of fusion of water is 6.01 kJ/mol. The heat capacity of liquid water is 75.3 J/mol ∙ K. The
conversion of 50.0 g of ice at 0.00 °C to liquid water at 0.00°C requires ________ kJ of heat.
A) 6.01
B) 16.7
C) 75.3
D) 17.2
E) Insufficient data are given.