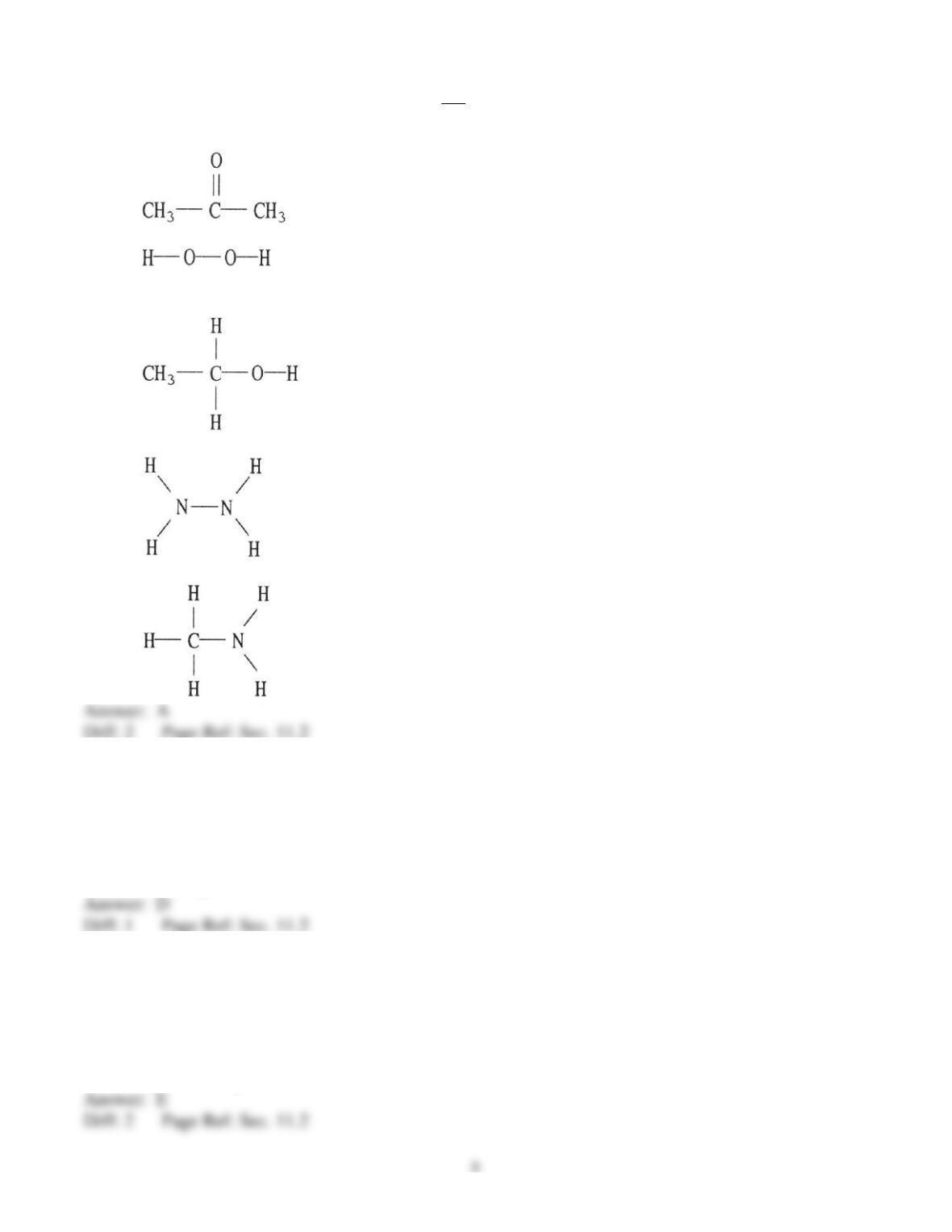
39) What type(s) of intermolecular forces exist between Br2 and CCl4?
A) dispersion forces
B) dispersion forces and ion-dipole
C) dispersion forces and dipole-dipole
D) dispersion forces, ion-dipole, and dipole-dipole
E) None. Since both are gases at room temperature, they do not interact with each other.
40) What type(s) of intermolecular forces exist between Cl2 and CO3-2?
A) dispersion forces
B) dispersion forces and ion-dipole
C) dispersion forces, ion-dipole, and induced dipole - induced dipole
D) dispersion forces and ion-induced dipole
E) dispersion forces, ion-dipole, dipole-dipole, and ion-induced dipole
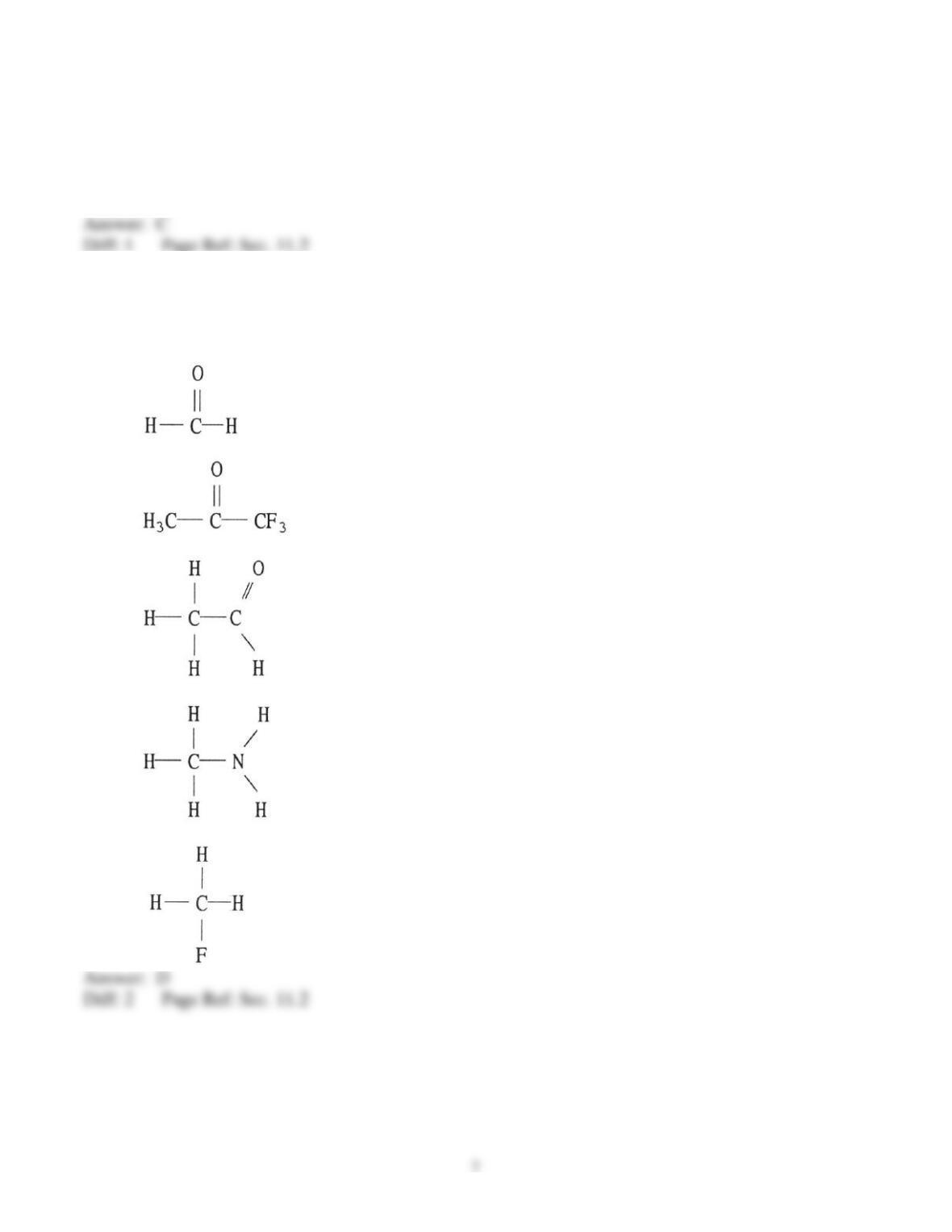
41) What types of intermolecular forces exist between NH3 and CBr4?
A) dispersion forces and dipole-dipole forces
B) dispersion forces and dipole-induced dipole forces
C) dispersion forces and hydrogen bonds
D) dispersion forces, hydrogen bonds, and induced dipole-induced dipole forces
E) dispersion forces, hydrogen bonds, and dipole-induced dipole forces
42) What types of intermolecular forces exist between PH3 and N2?
A) dispersion forces and dipole-dipole forces
B) dispersion forces and dipole-induced dipole forces
C) dispersion forces and hydrogen bonds
D) dispersion forces, hydrogen bonds, and induced dipole-induced dipole forces
E) dispersion forces, hydrogen bonds, and dipole-induced dipole forces
43) __________ is the energy required to expand the surface area of a liquid by a unit amount of area.
A) Viscosity
B) Surface tension
C) Volatility
D) Meniscus
E) Capillary action