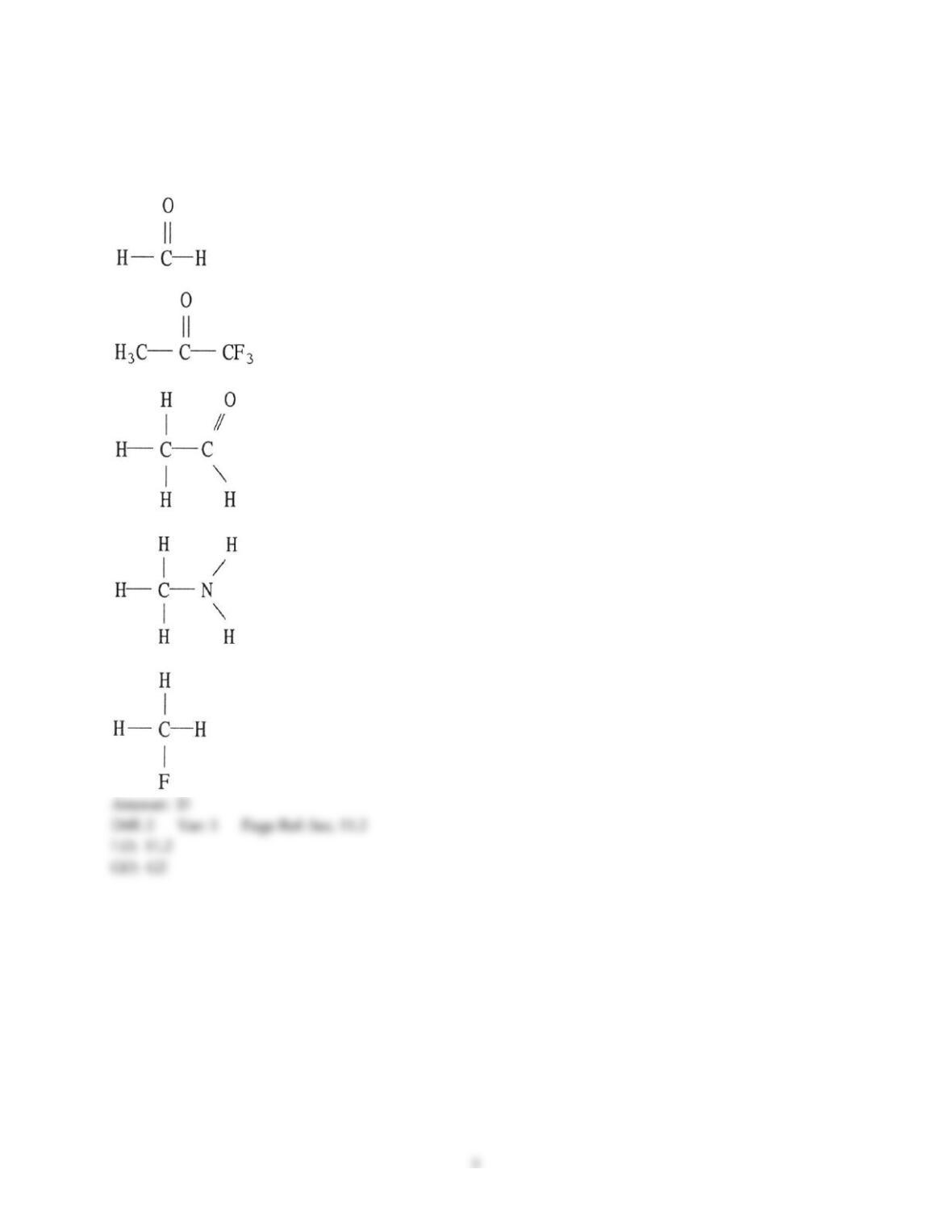
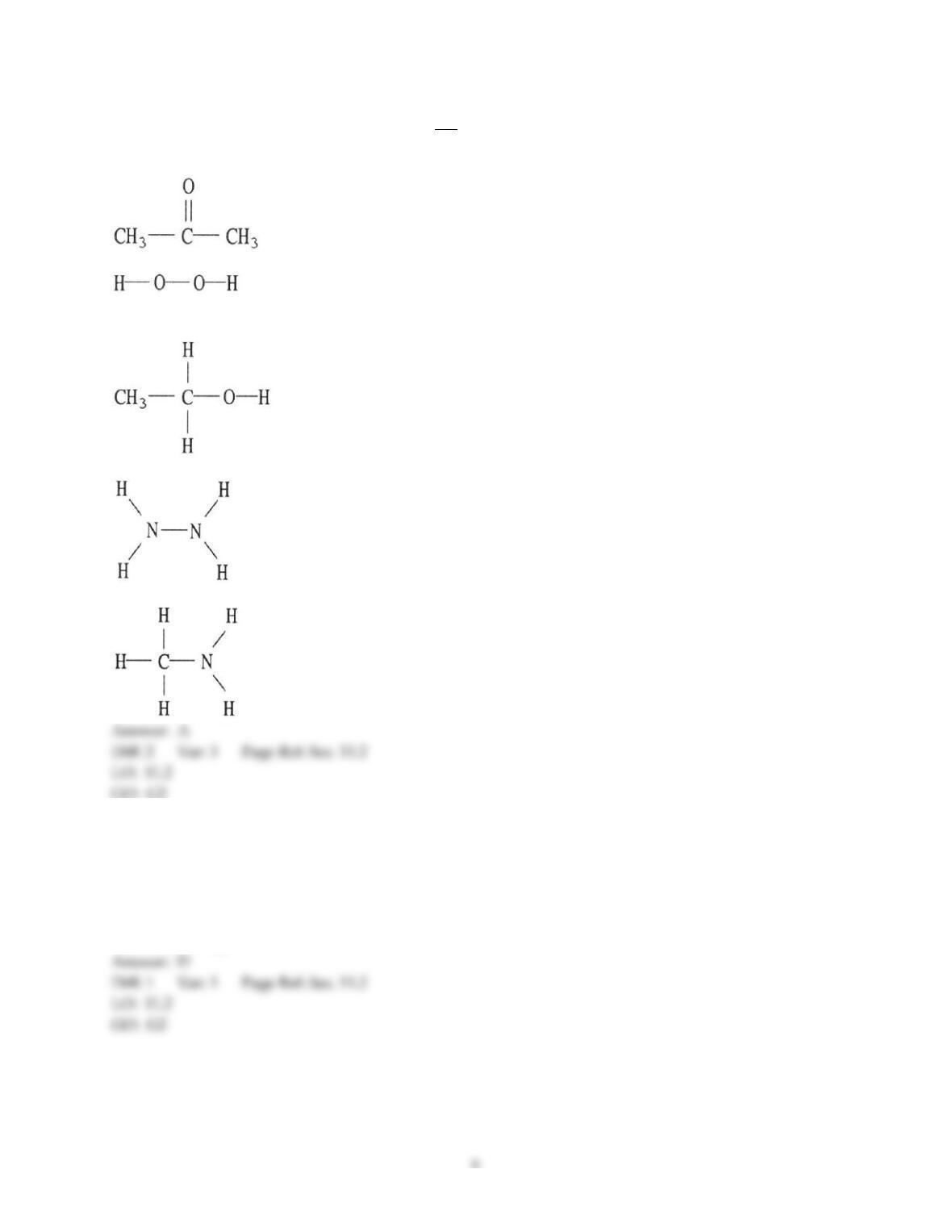
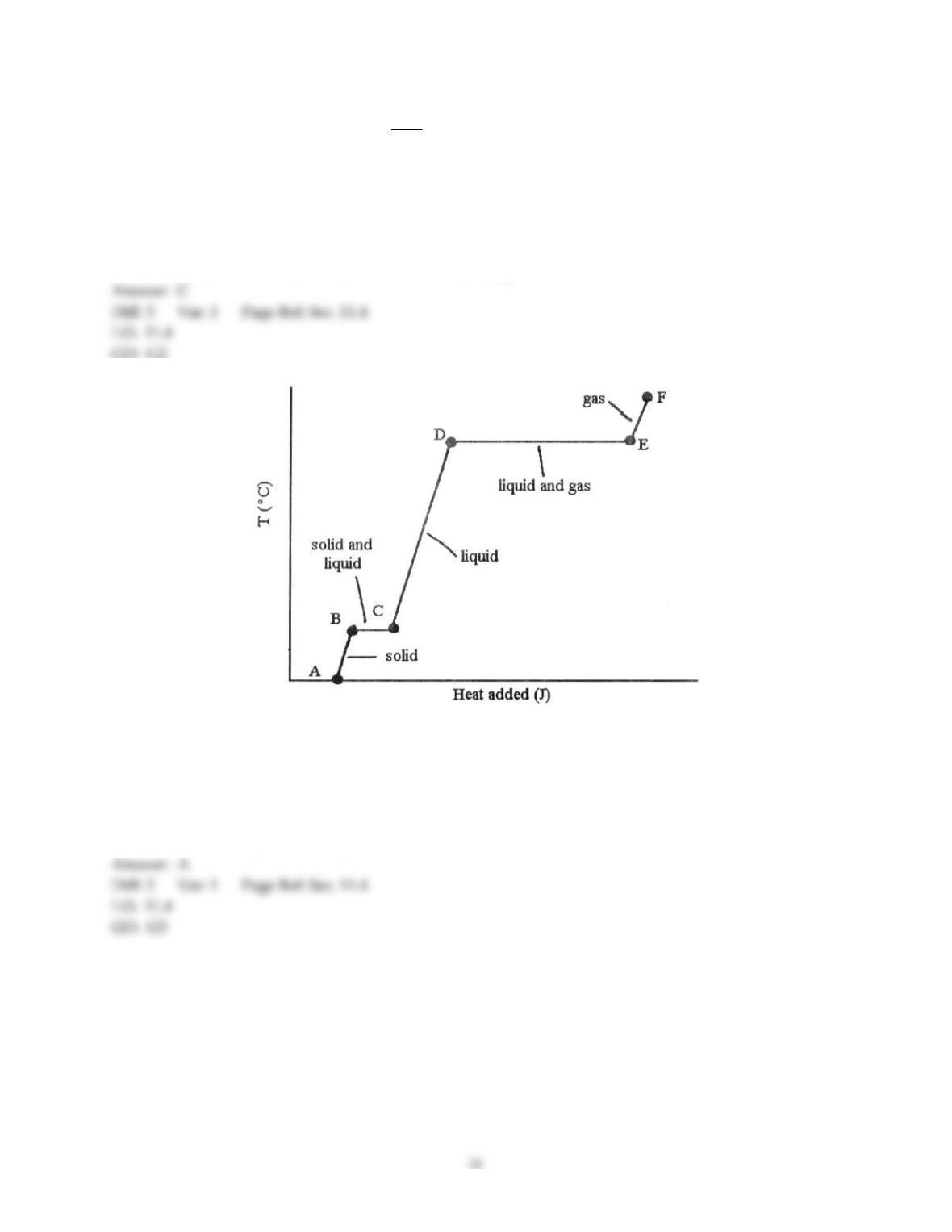
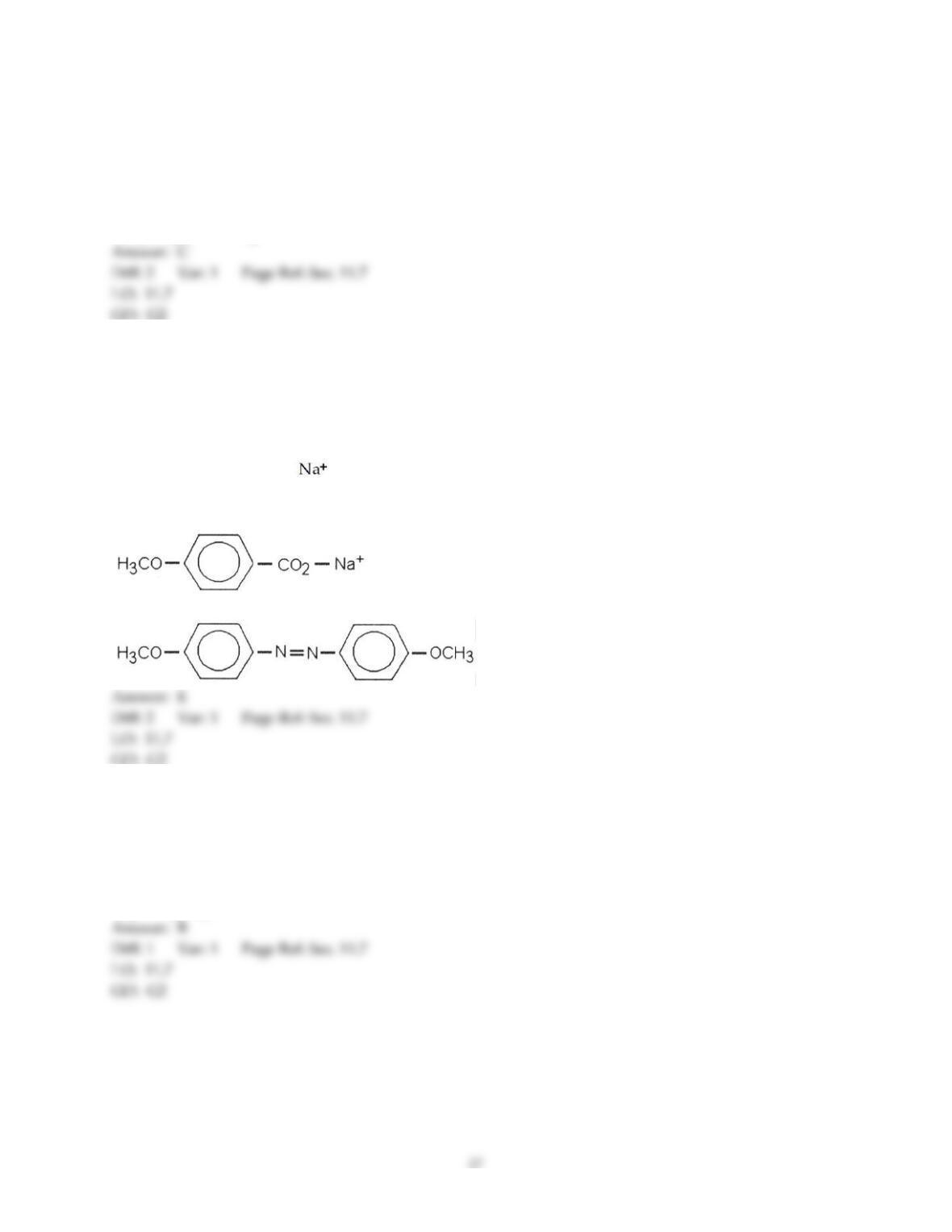
Chemistry: The Central Science, 13e (Brown et al.)
Chapter 11 Liquids and Intermolecular Forces
11.1 Multiple-Choice Questions
1) Crystalline solids ________.
A) have their particles arranged randomly
B) have highly ordered structures
C) are usually very soft
D) exist only at high temperatures
E) exist only at very low temperatures
2) In liquids, the attractive intermolecular forces are ________.
A) very weak compared with kinetic energies of the molecules
B) strong enough to hold molecules relatively close together
C) strong enough to keep the molecules confined to vibrating about their fixed lattice points
D) not strong enough to keep molecules from moving past each other
E) strong enough to hold molecules relatively close together but not strong enough to keep molecules
from moving past each other
3) As a gaseous element condenses, the atoms become ________ and they have ________ attraction for one
another.
A) more separated, more
B) more separated, less
C) closer together, more
D) closer together, less
E) larger, greater
4) A gas is ________ and assumes ________ of its container, whereas a liquid is ________ and assumes
________ of its container.
A) compressible, the volume and shape, not compressible, the shape of a portion
B) compressible, the shape, not compressible, the volume and shape
C) compressible, the volume and shape, compressible, the volume
D) condensed, the volume and shape, condensed, the volume and shape
E) condensed, the shape, compressible, the volume and shape