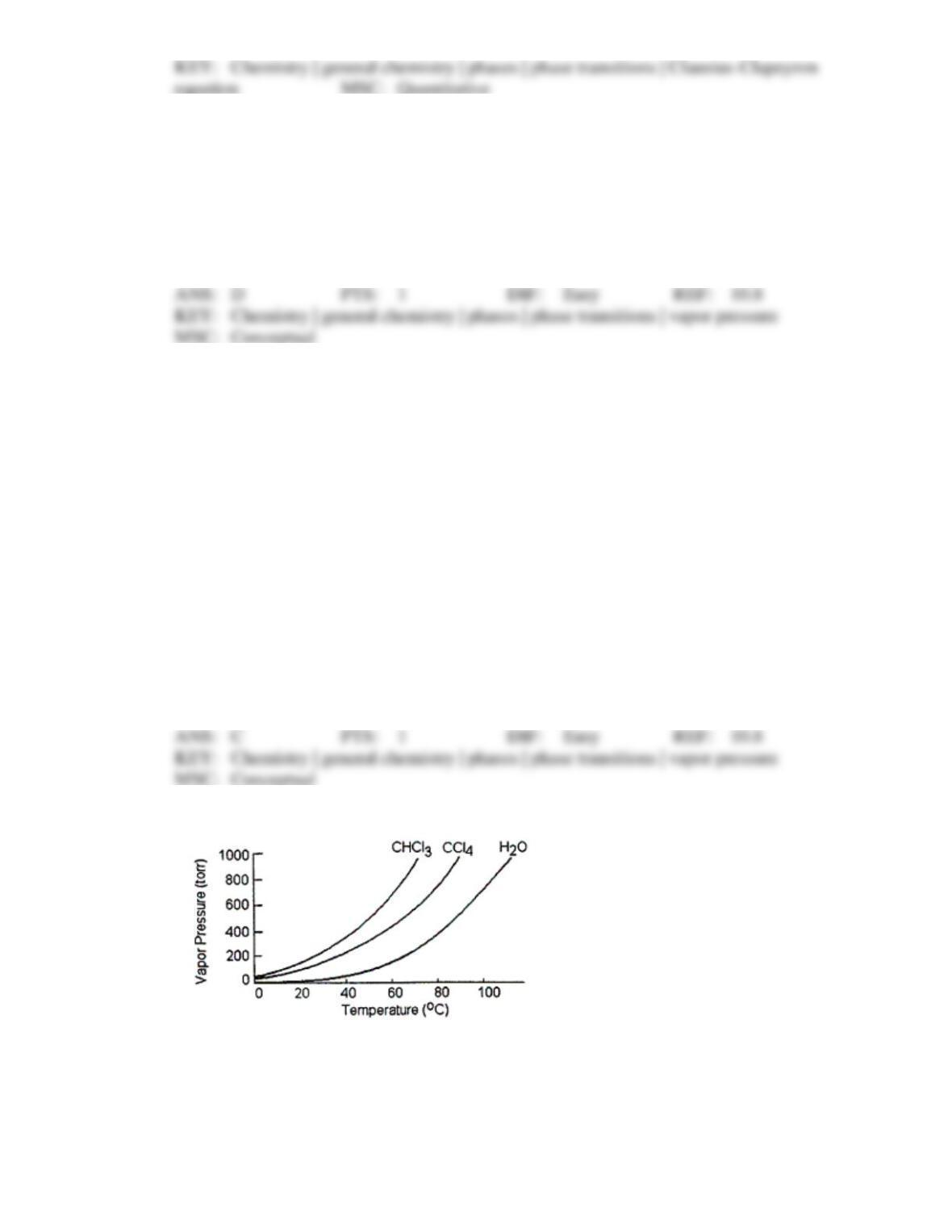
106. A certain substance, X, has a triple-point temperature of 20°C at a pressure of 2.0 atm.
Which one of the statements (A-D) cannot possibly be true?
X can exist as a liquid above 20°C.
X can exist as a solid above 20°C.
Liquid X can exist as a stable phase at 25°C, 1 atm.
Both liquid and solid X have the same vapor pressure at 20°C.
All of the statements (A-D) could be true.
107. Which statement regarding water is true?
Energy must be given off in order to break down the crystal lattice of ice to a
liquid.
Hydrogen bonds are stronger than covalent bonds.
Liquid water is less dense than solid water.
Only covalent bonds are broken when ice melts.
All of the statements (A–D) are false.
108. The triple point of iodine is at 90 torr and 115°C. This means that liquid I2
cannot exist at 1 atmosphere pressure
cannot have a vapor pressure less than 90 torr
can exist at pressure of 10 torr
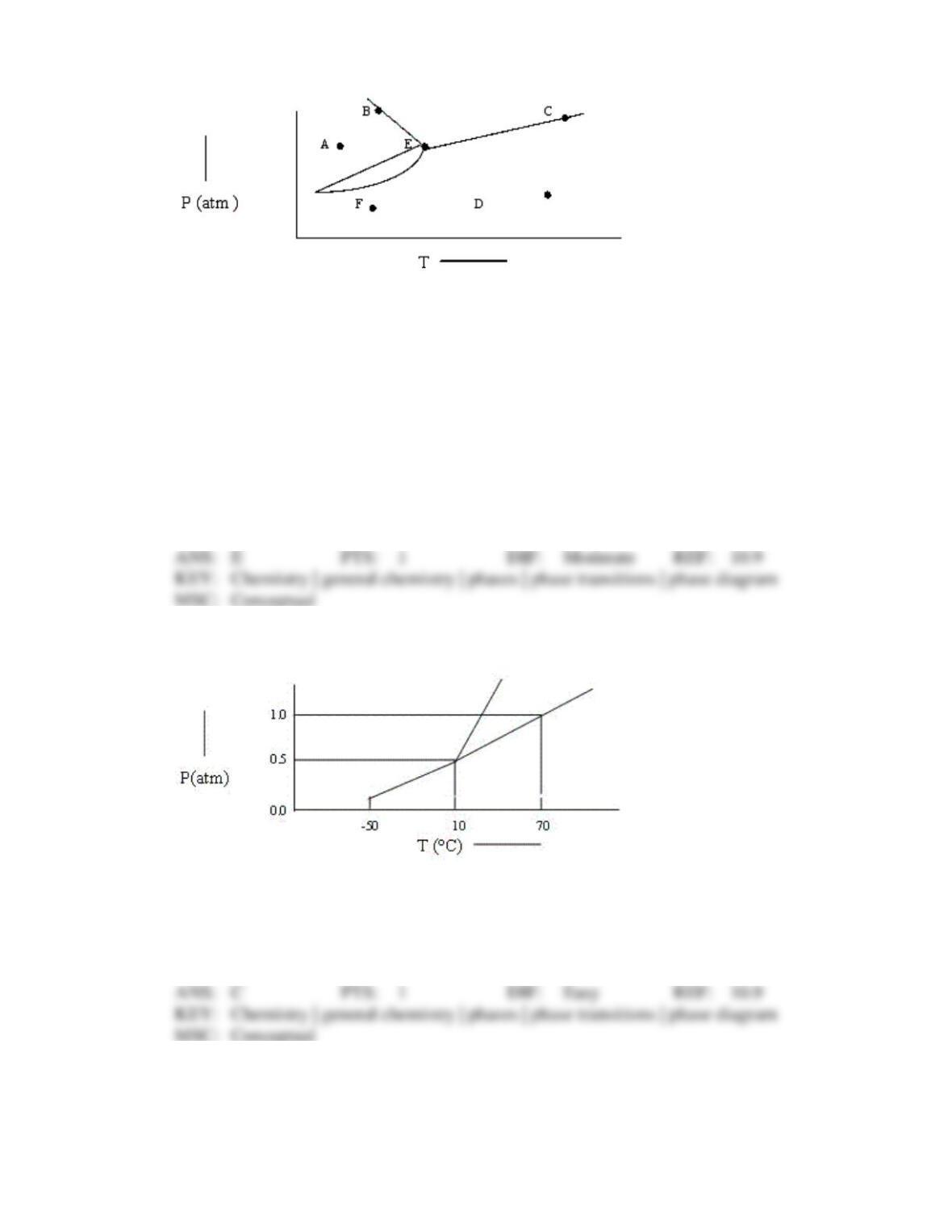
109. The triple point of CO2 is at 5.2 atm and –57°C. Under atmospheric conditions present in a
typical Boulder, Colorado, laboratory (P = 630 torr, T = 23°C), solid CO2 will:
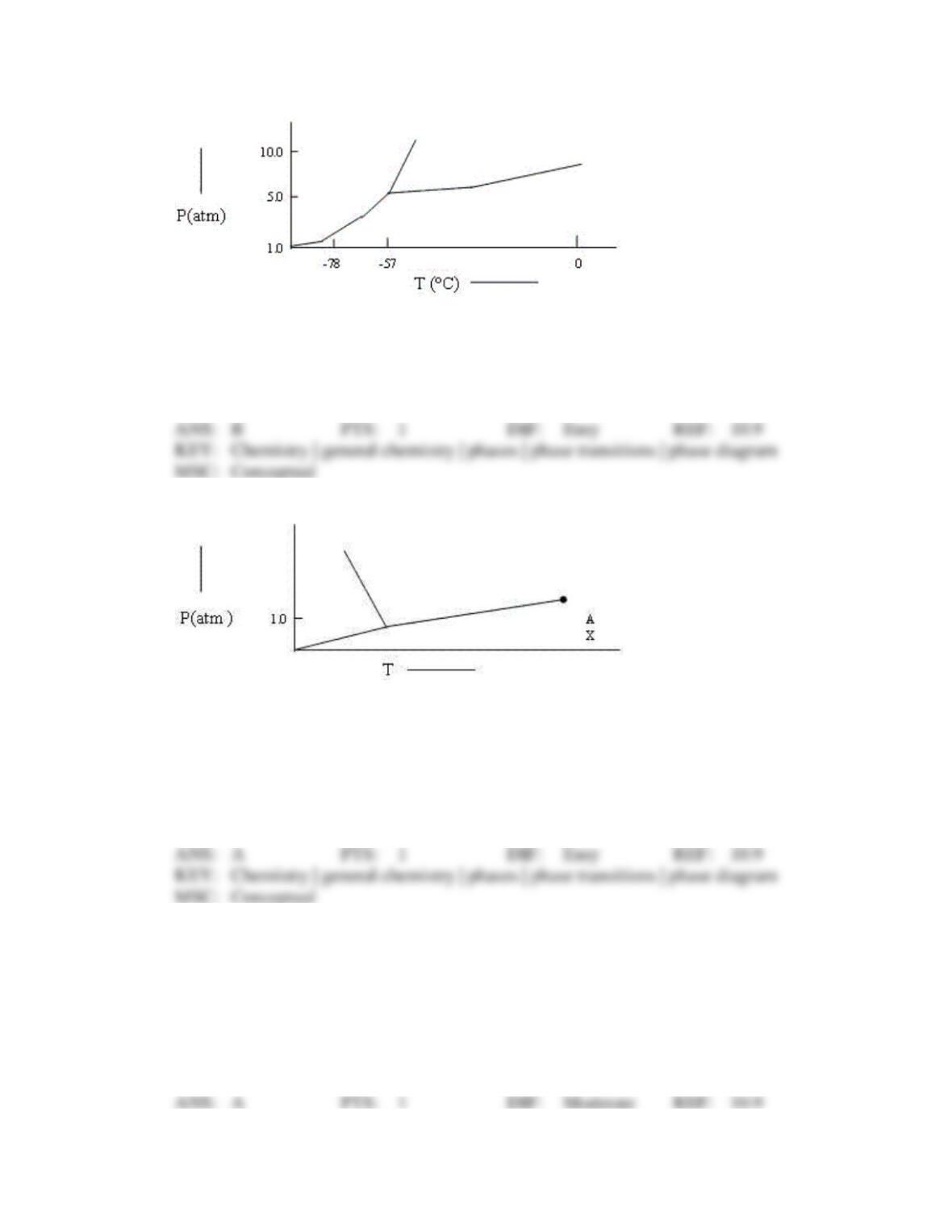
110. Choose the correct statement about the diagram below.