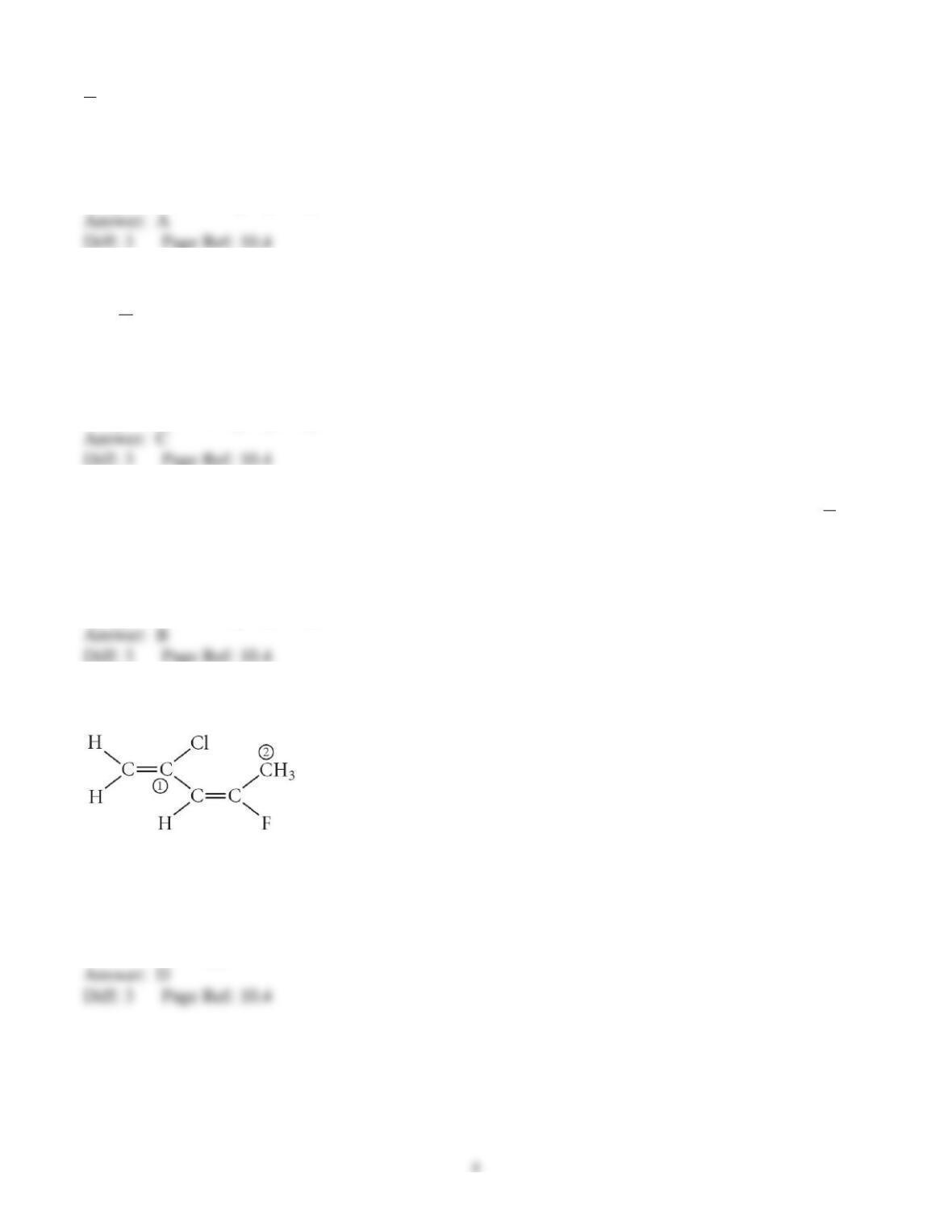
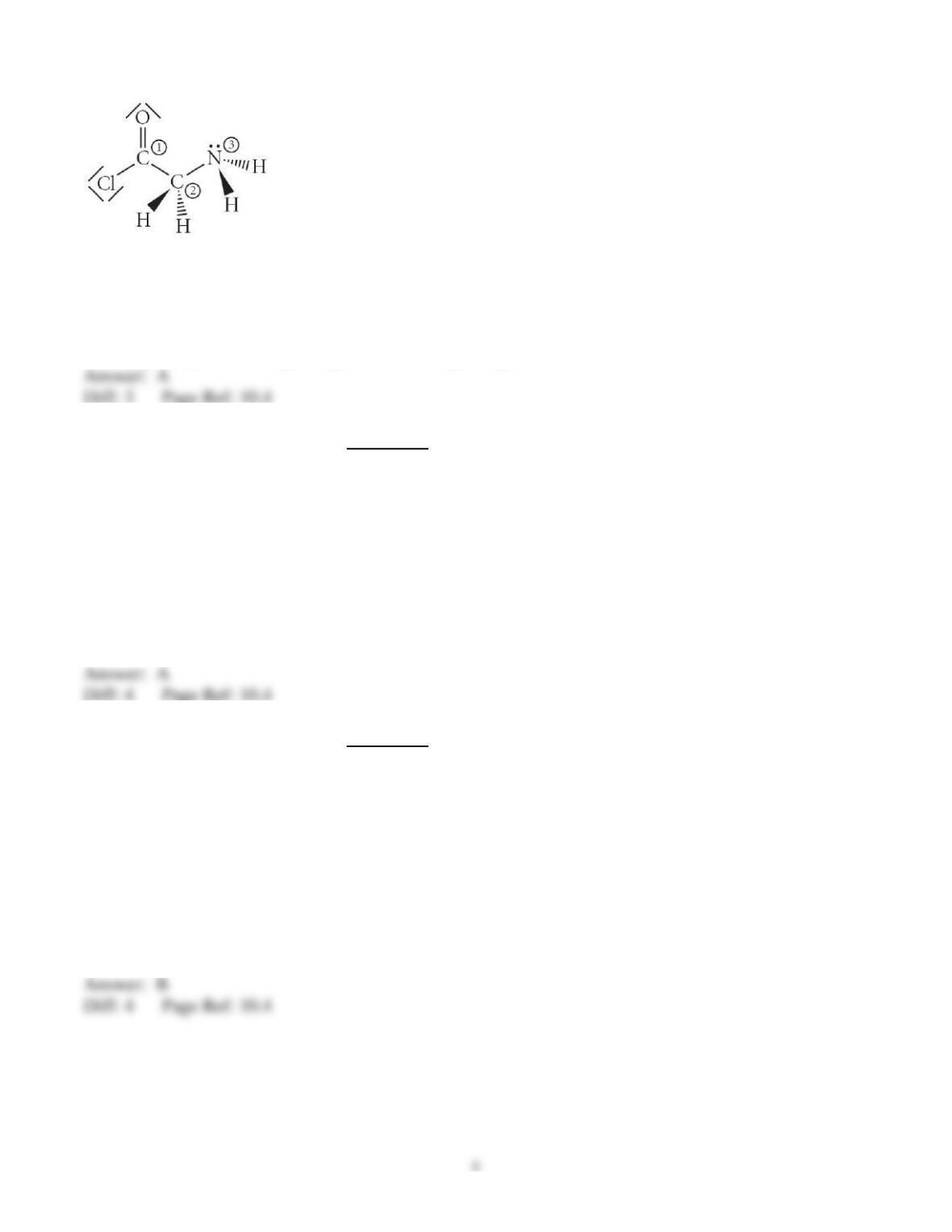
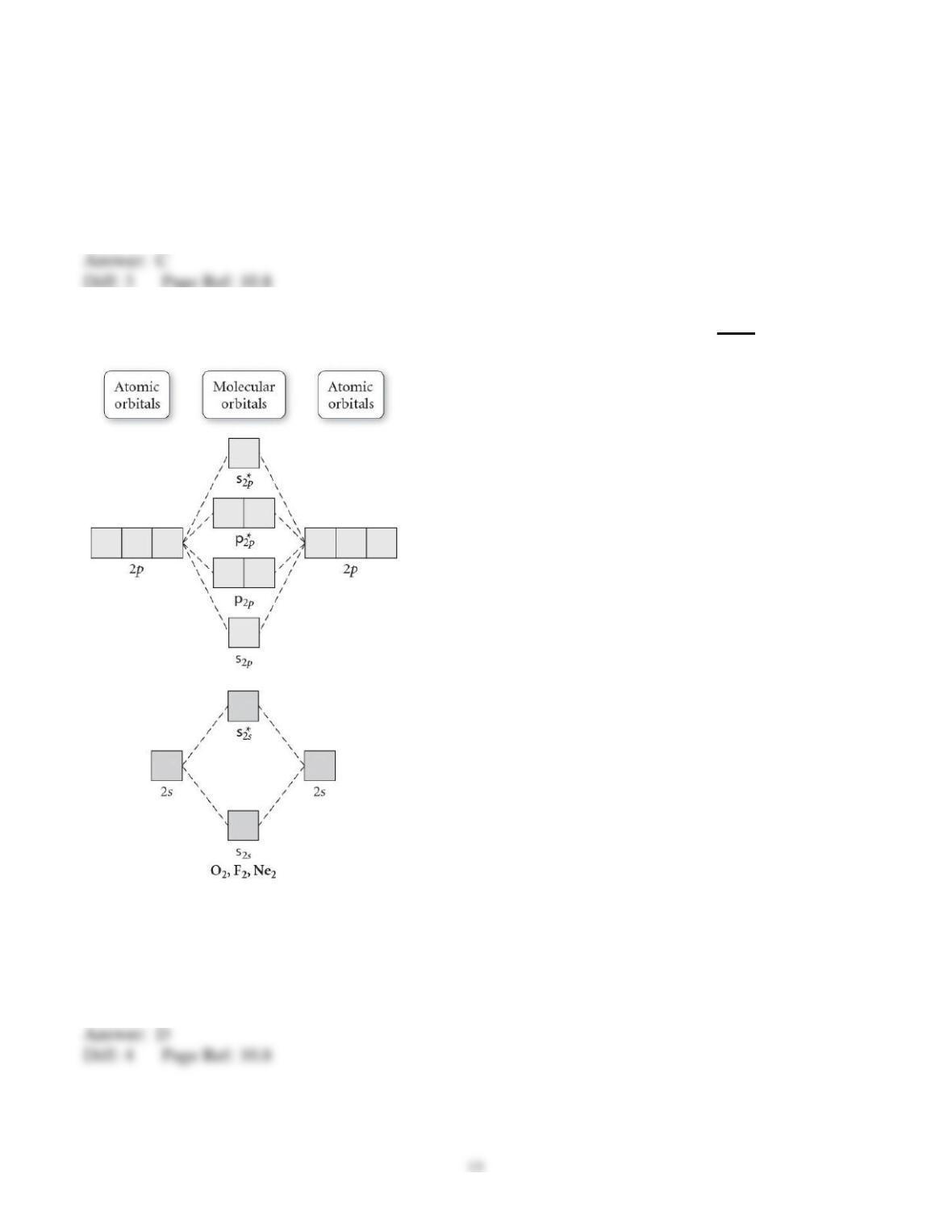
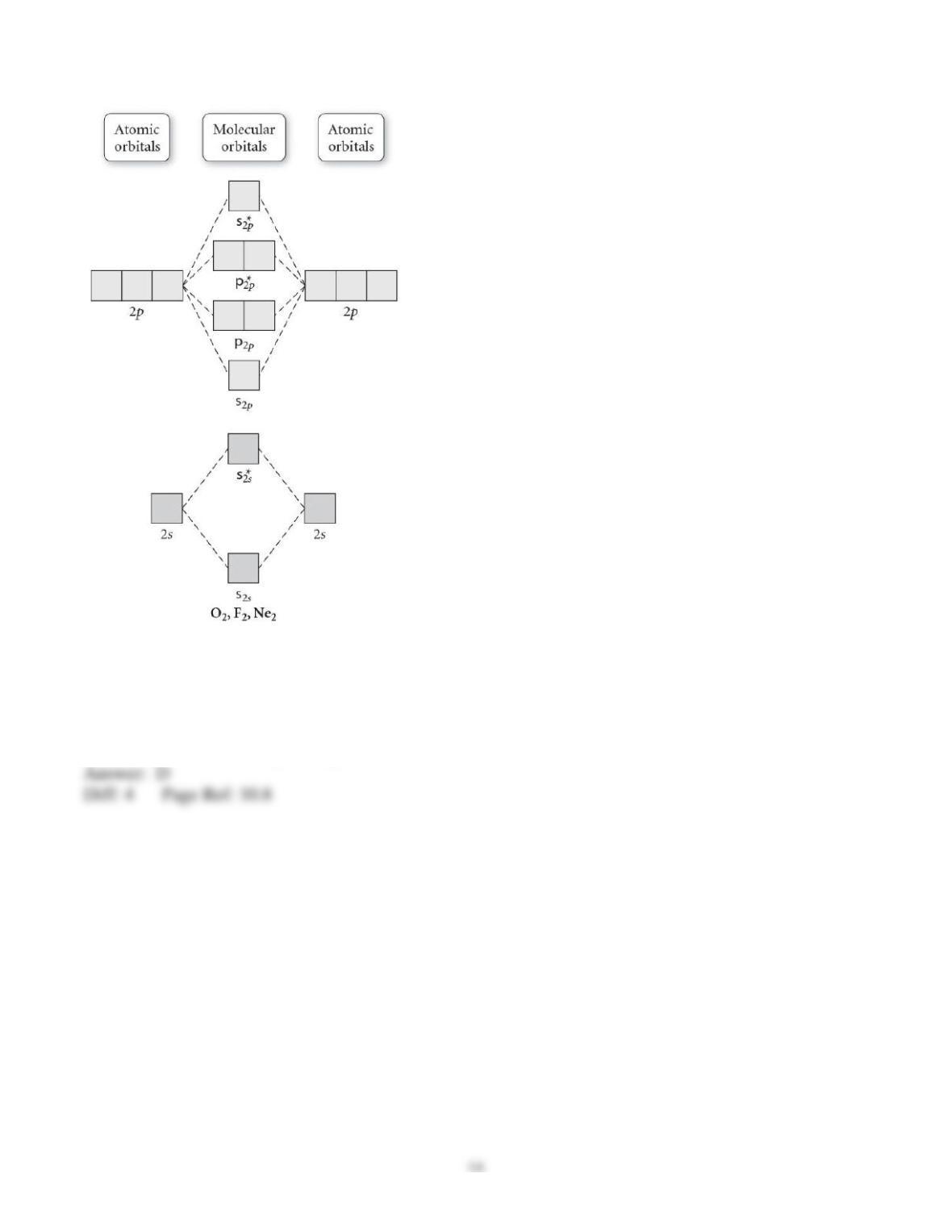
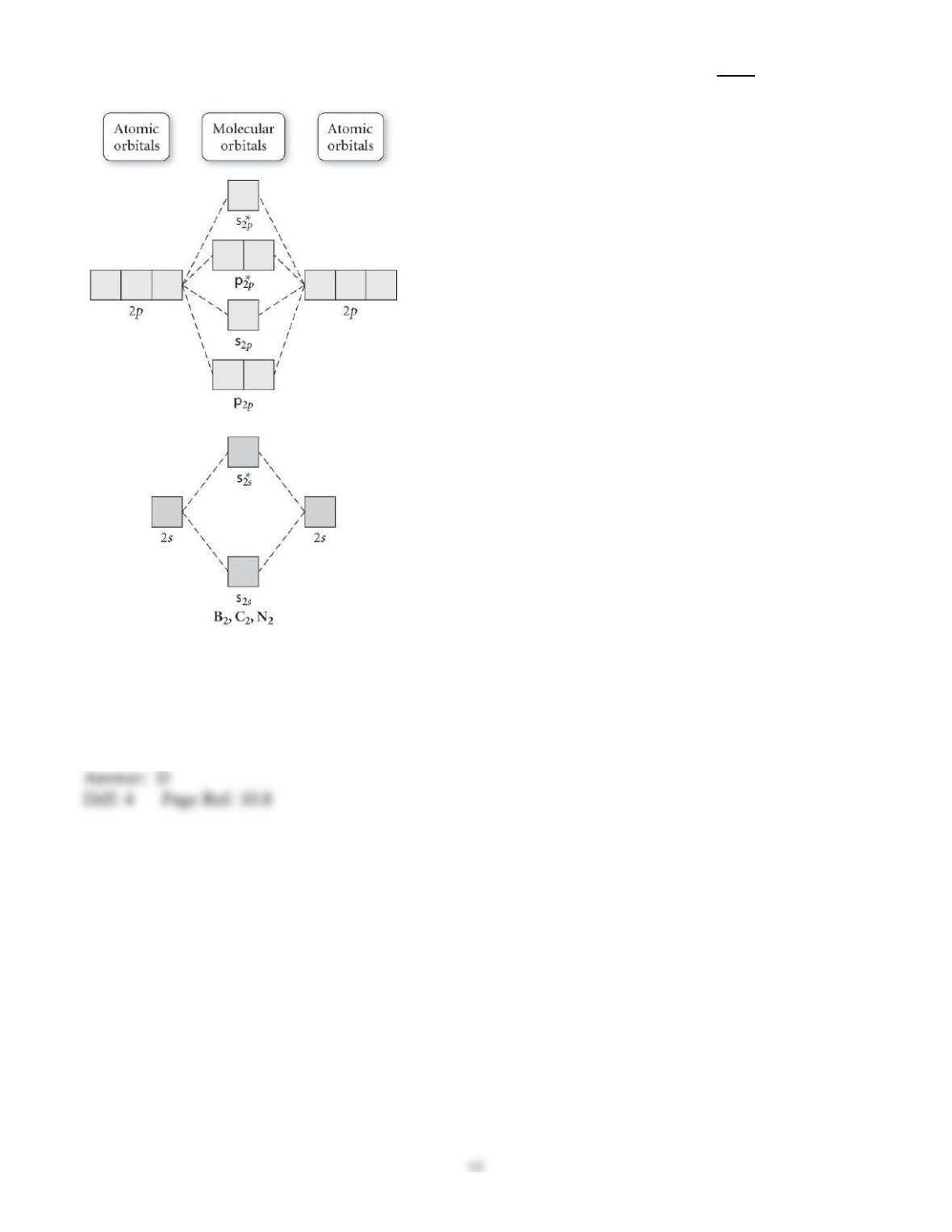
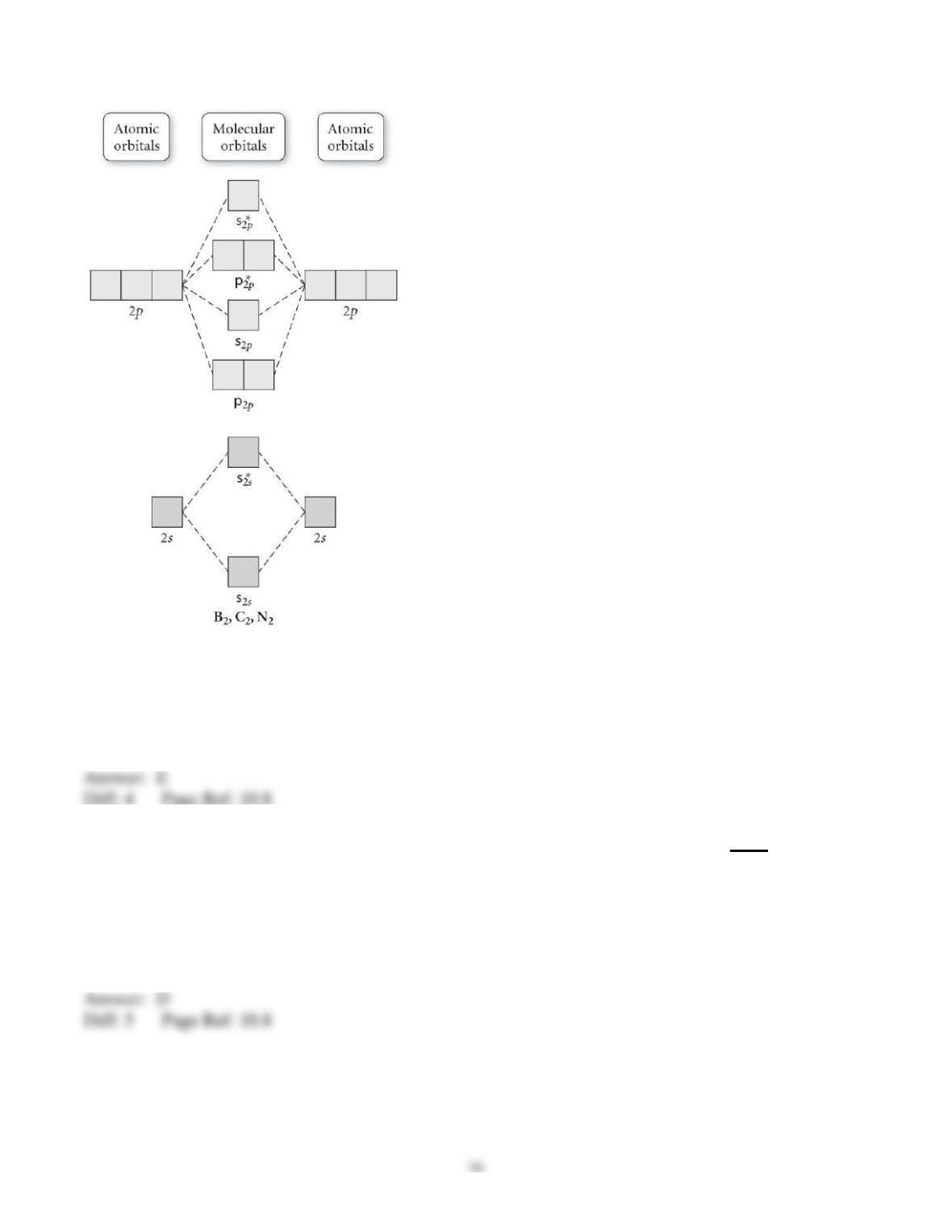
29) Determine the electron geometry (eg), molecular geometry (mg), and polarity of PCl3.
A) eg=tetrahedral, mg=bent, polar
B) eg=trigonal planar, mg=trigonal planar, nonpolar
C) eg=linear, mg=linear, nonpolar
D) eg=tetrahedral, mg=trigonal pyramidal, polar
E) eg=trigonal pyramidal, mg=trigonal pyramidal, polar
30) Determine the electron geometry, molecular geometry and polarity of SF6.
A) eg=trigonal bipyramidal, mg=trigonal bipyramidal, nonpolar
B) eg=tetrahedral, mg=tetrahedral, polar
C) eg=trigonal bipyramidal, mg=see-saw, polar
D) eg=octahedral, mg=trigonal bipyramidal, nonpolar
E) eg=octahedral, mg=octahedral, nonpolar
31) Determine the electron geometry (eg), molecular geometry(mg) and polarity of XeO3.
A) eg=trigonal planar, mg=trigonal planar, nonpolar
B) eg=tetrahedral, mg=trigonal pyramidal, polar
C) eg=trigonal planar, mg=trigonal pyramidal, polar
D) eg=trigonal bipyramidal, mg=trigonal planar, nonpolar
E) eg=octahedral, mg=tetrahedral, nonpolar
32) Determine the electron geometry, molecular geometry and polarity of HBrO2.
A) eg=trigonal bipyramidal, mg=trigonal planar, nonpolar
B) eg=octahedral, mg=square planar, nonpolar
C) eg=tetrahedral, mg=trigonal pyramidal, polar
D) eg=tetrahedral, mg=linear, nonpolar
E) eg=linear, mg=linear, polar
33) Place the following in order of increasing dipole moment.
I. BCl3 II. BIF2 III. BClF2
A) I < II = III
B) II < III < I
C) I < II < III
D) II < I < III
E) I < III < II