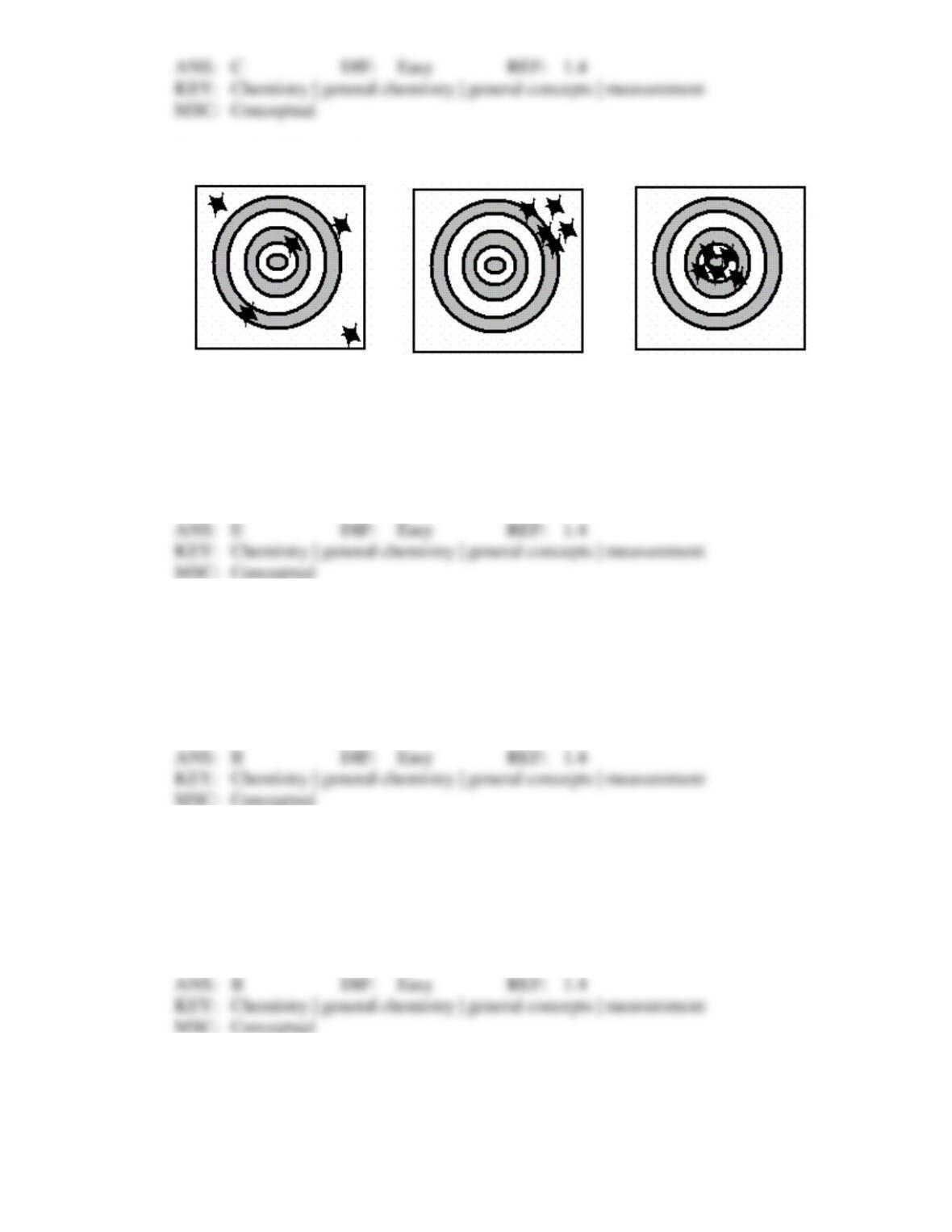
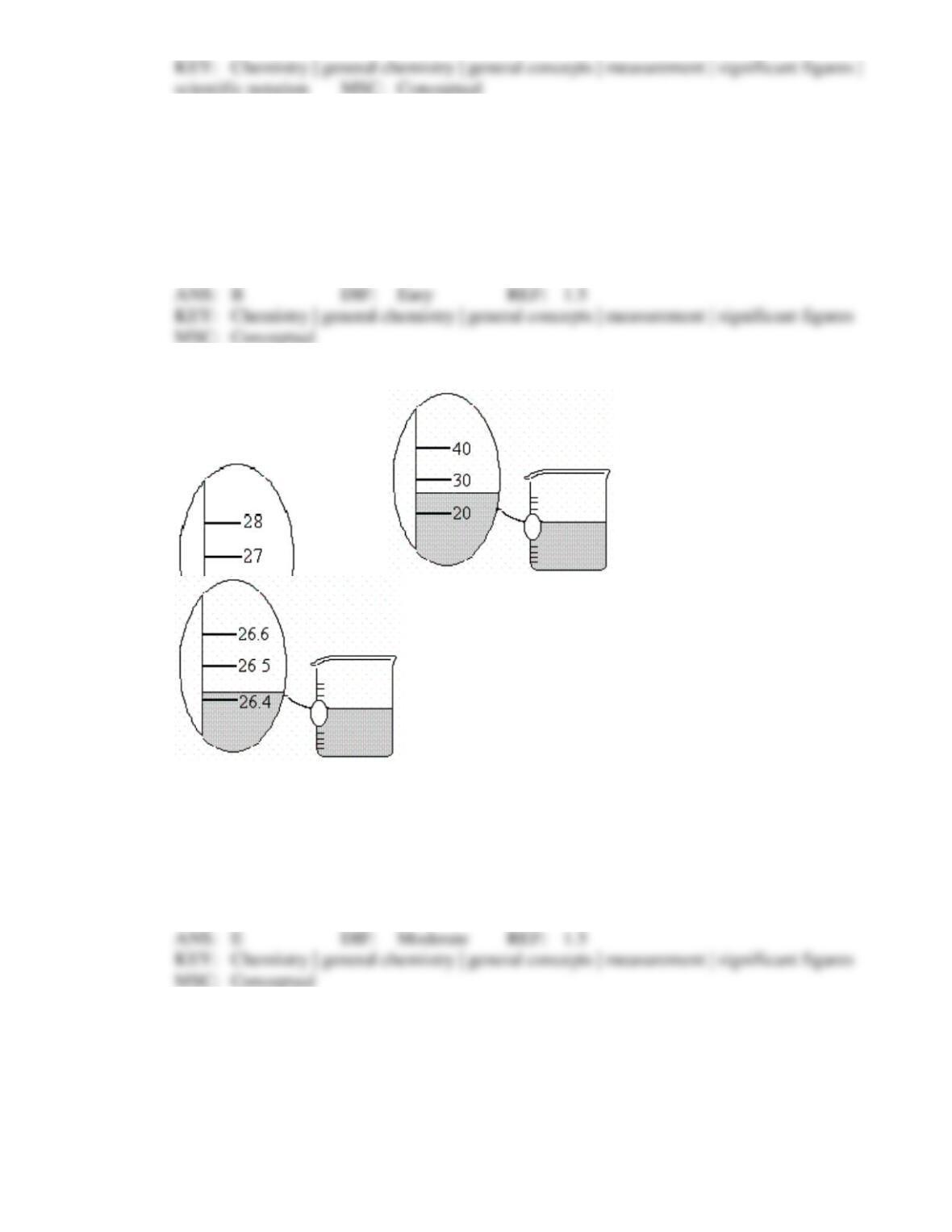
Chapter 1: Chemical Foundations
1. Which of the following is an example of a quantitative observation?
The piece of metal is longer than the piece of wood.
Solution 1 is much darker than solution 2.
The liquid in beaker A is blue.
The temperature of the liquid is 60°C.
At least two of the above (A-D) are quantitative observations.
2. A quantitative observation
contains a number and a unit
does not contain a number
always makes a comparison
must be obtained through experimentation
3. Generally, observed behavior that can be formulated into a statement, sometimes
mathematical in nature, is called a(n)
4. The statement “The total mass of materials is not affected by a chemical change in those
materials” is called a(n)
5. A chemical theory that has been known for a long time becomes a law.