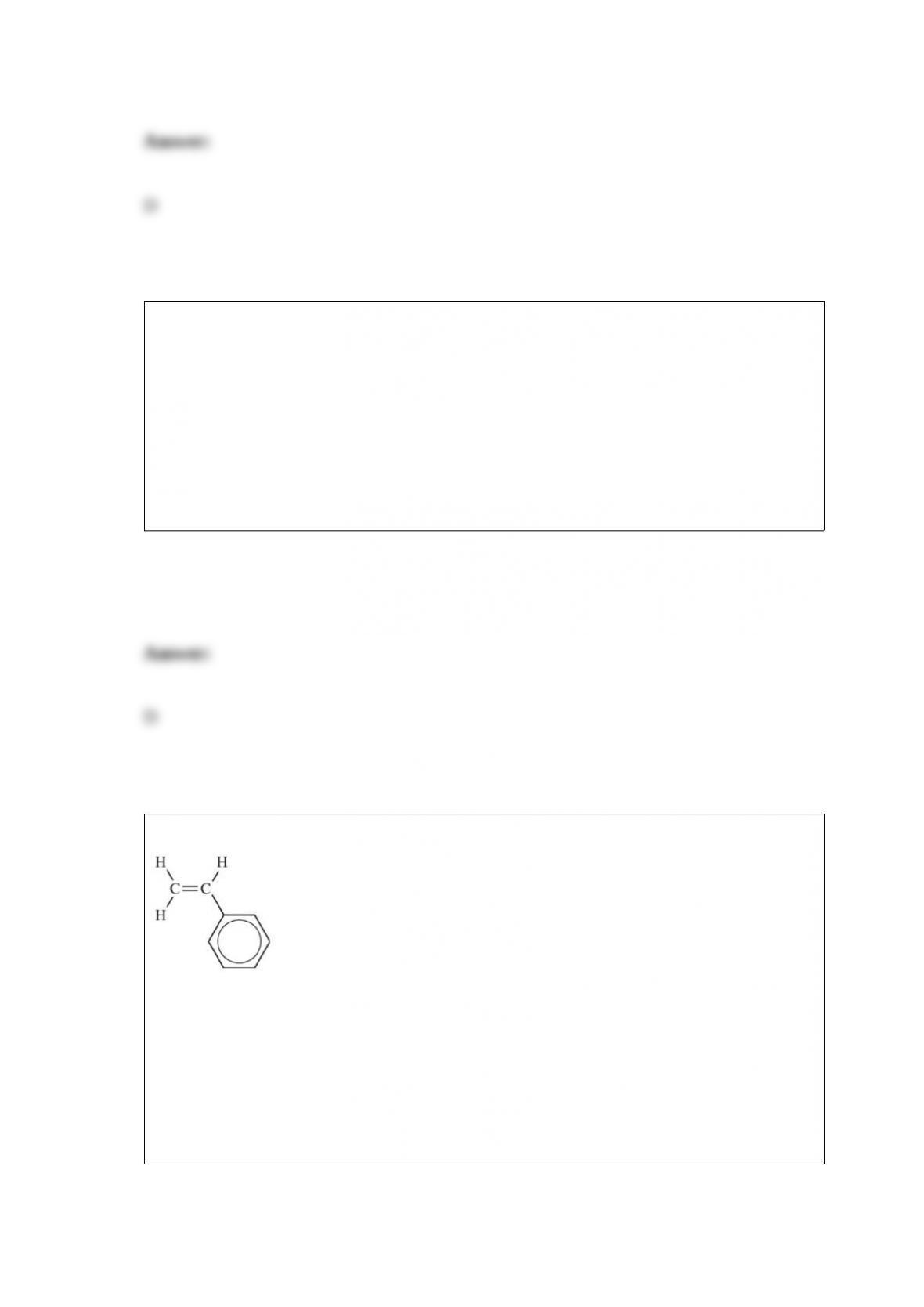
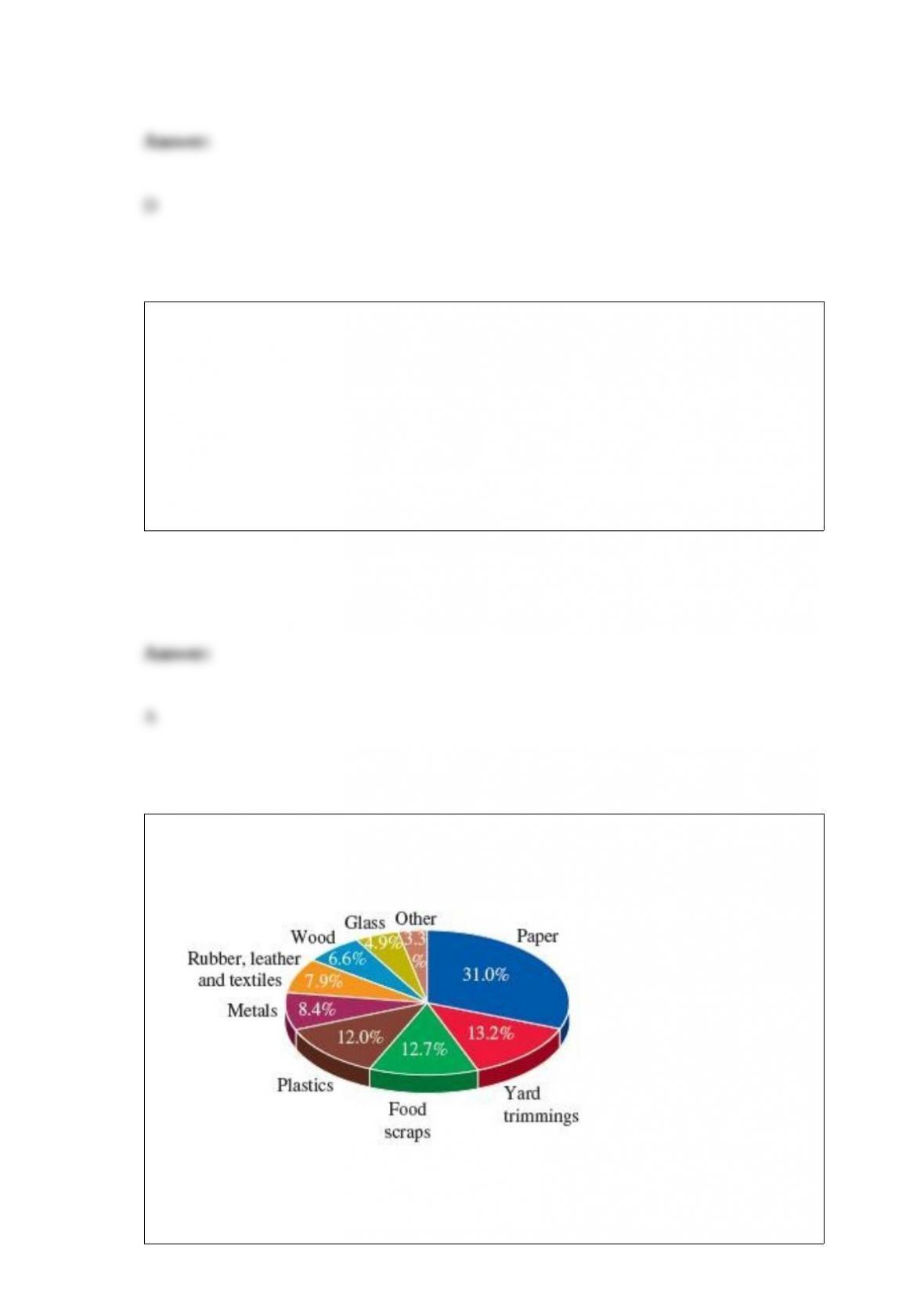
What is the current expert consensus concerning the role of acid rain on the health of
forests?
A. Acid rain is indisputably responsible for the declining health of many forests.
B. Acid rain is not responsible for any of the decline observed in many forests.
C. Acid rain weakens trees and the surrounding soil, leaving them susceptible to disease
and insects.
D. There is not sufficient evidence that acid rain has caused an appreciable decline in
the health of any forests.
Ozone in our atmosphere is important because it
A. absorbs some UV radiation.
B. helps trees grow.
C. reacts with excess CO2.
D. reflects IR radiation.
The Industrial Revolution is a key point in the Earth's atmospheric history because