1) Which of the following traits characterizes the alkali metals?
A) very high melting point
B) existence as diatomic molecules
C) formation of dianions
D) the lowest first ionization energies in a period
E) the smallest atomic radius in a period
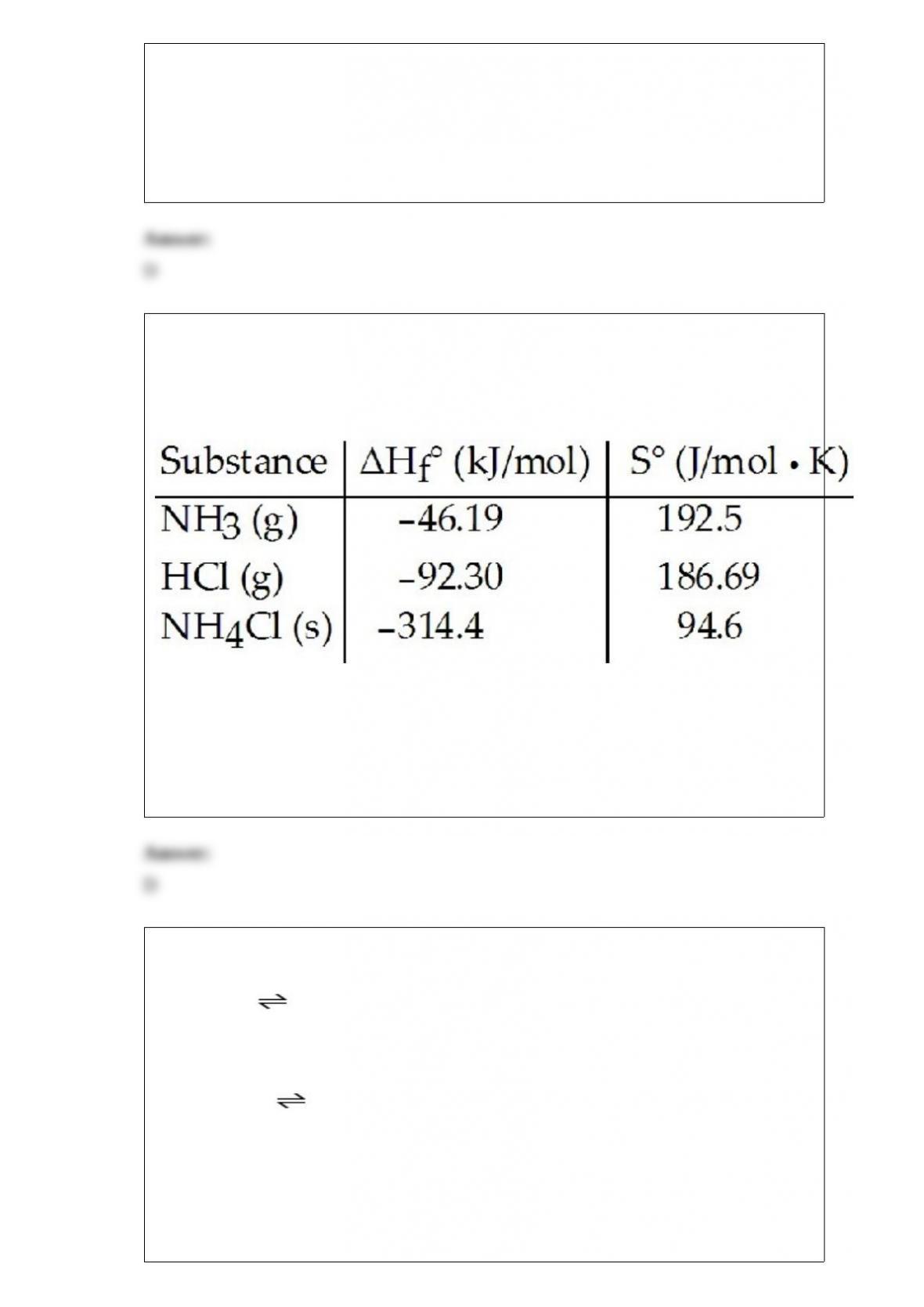
2) Consider the reaction:
NH3 (g) + HCl (g) --> NH4Cl (s)
Given the following table of thermodynamic data,
determine the temperature (in oC) above which the reaction is nonspontaneous.
A) This reaction is spontaneous at all temperatures.
B) 618.1
C) 432.8
D) 345.0
E) 1235
3) The value of Keq for the following reaction is 0.26:
A (g) + B (g) C (g) + D (g)
The value of Keq at the same temperature for the reaction below is ________.
2A (g) + 2B (g) 2C (g) + 2D (g)
A) 0.068
B) 0.52
C) 1.2
D) 0.065
E) 0.26