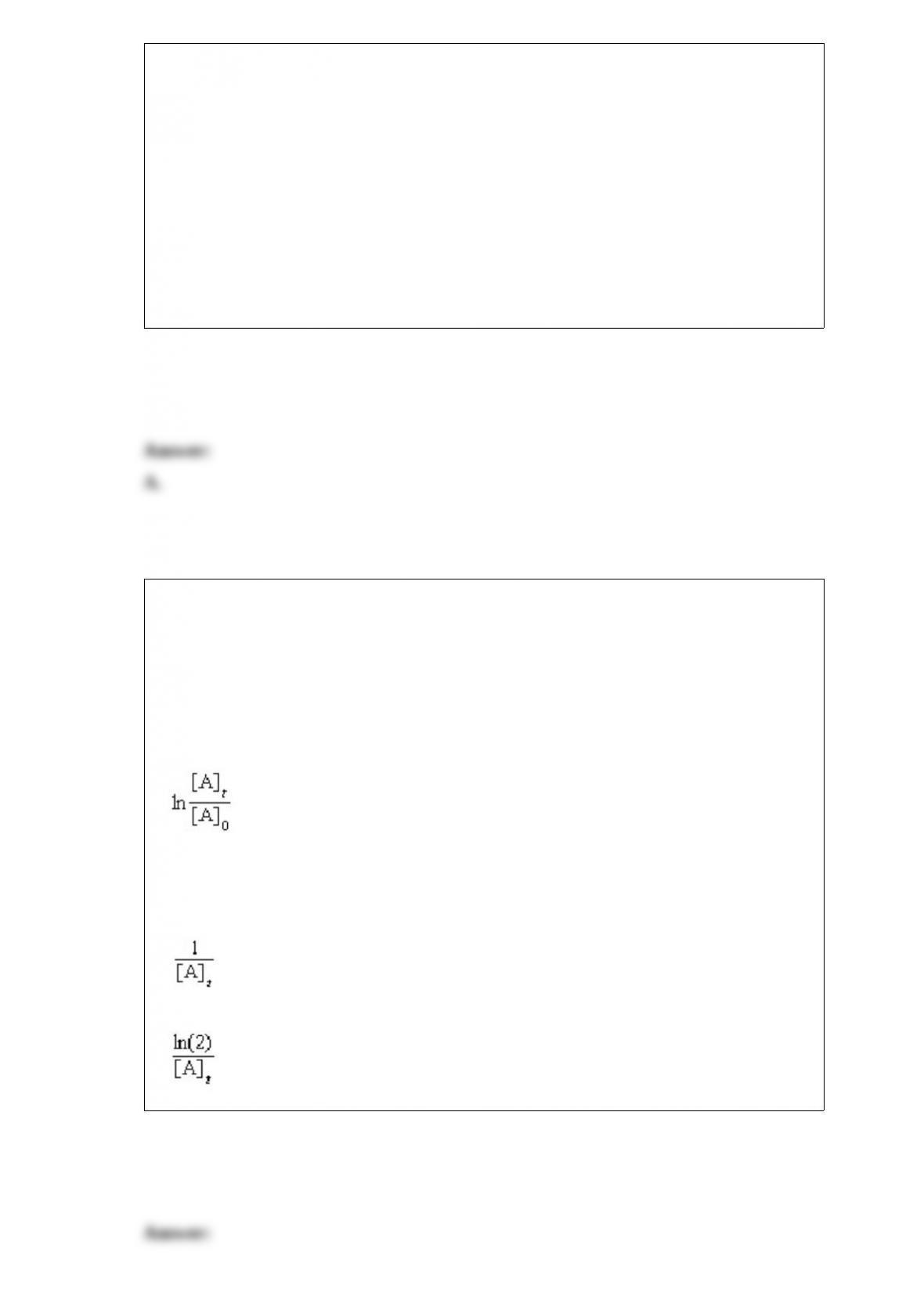
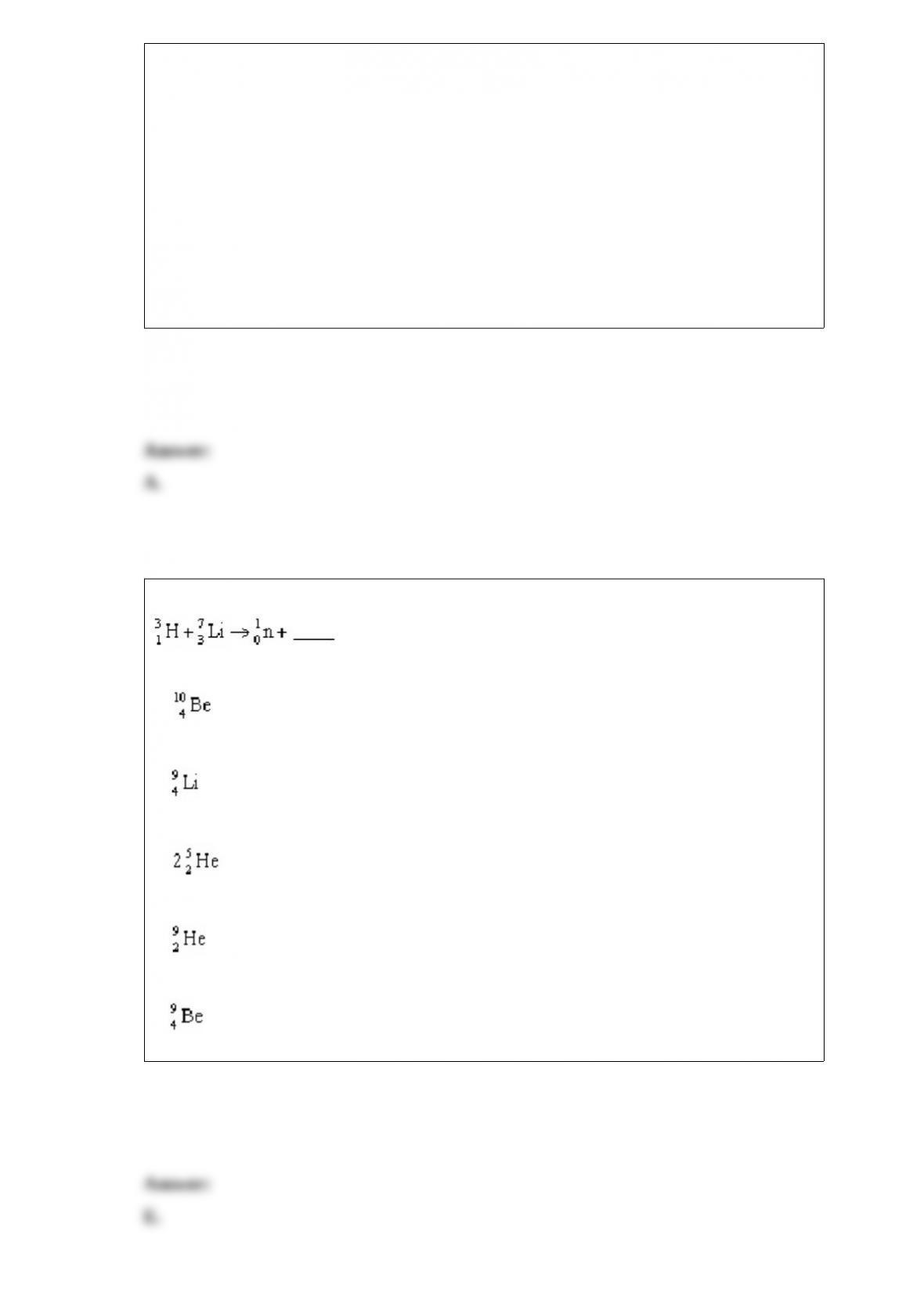
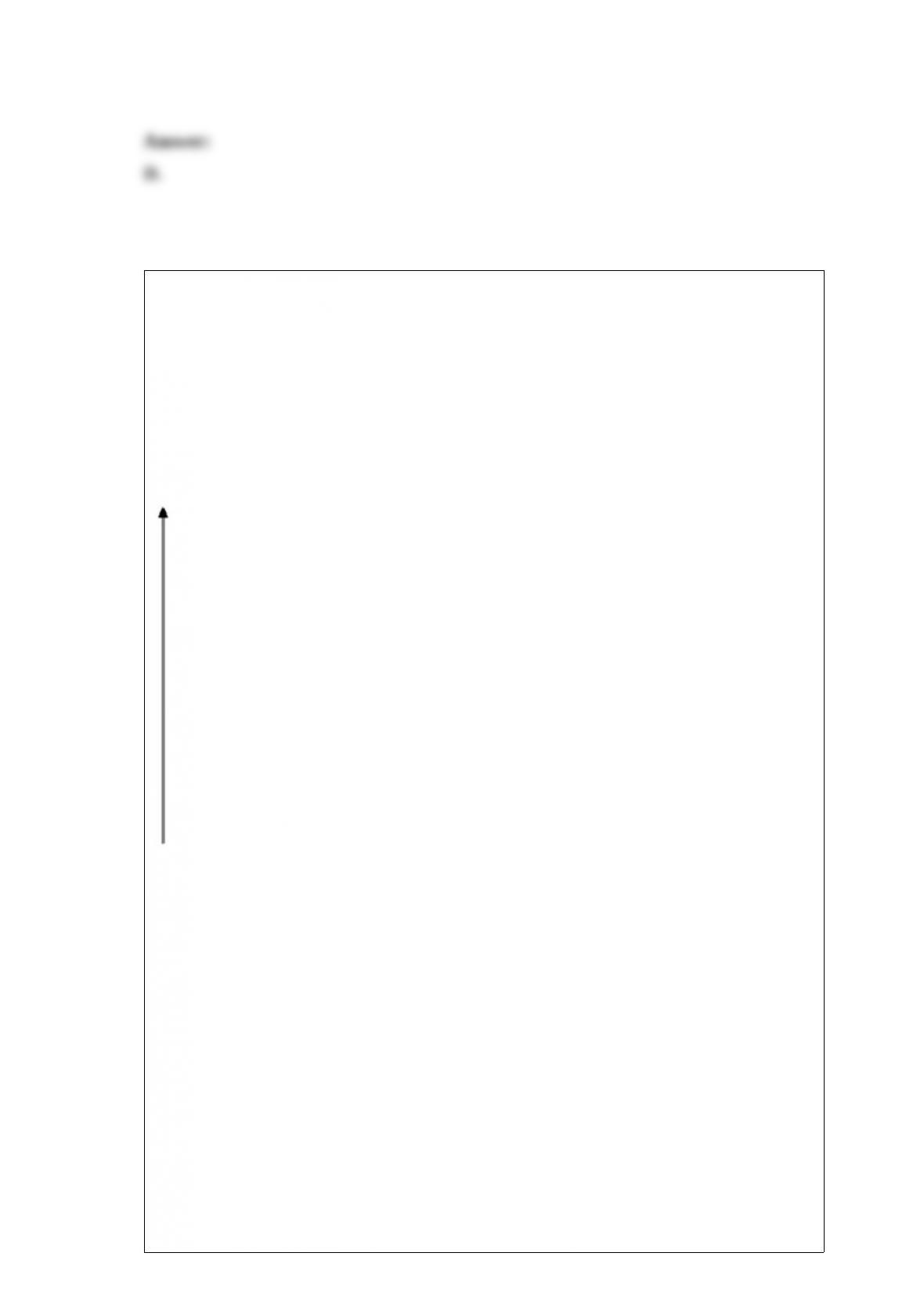
What is the oxidation number of each atom in sodium hydrogen carbonate, NaHCO3?
A.Na = +1, H = "1, C = +6, O = "2
B.Na = +1, H = +1, C = +4, O = "2
C.Na = +1, H = "1, C = +2, O = "2
D.Na = "1, H = +1, C = 0, O = "2
E.Na = 0, H = 0, C = 0, O = 0
Round 0.00680483 to 3 significant figures.
A.0.00
B.0.007
C.0.00680
D.0.006804
E.0.006805
Calcium carbonate, or limestone, is relatively insoluble in water. At 25 C, only 5.8 mg
will dissolve in 1.0 liter of water. What volume of water is needed to dissolve 5.0 g of
calcium carbonate?
A.4.6 10"3 L