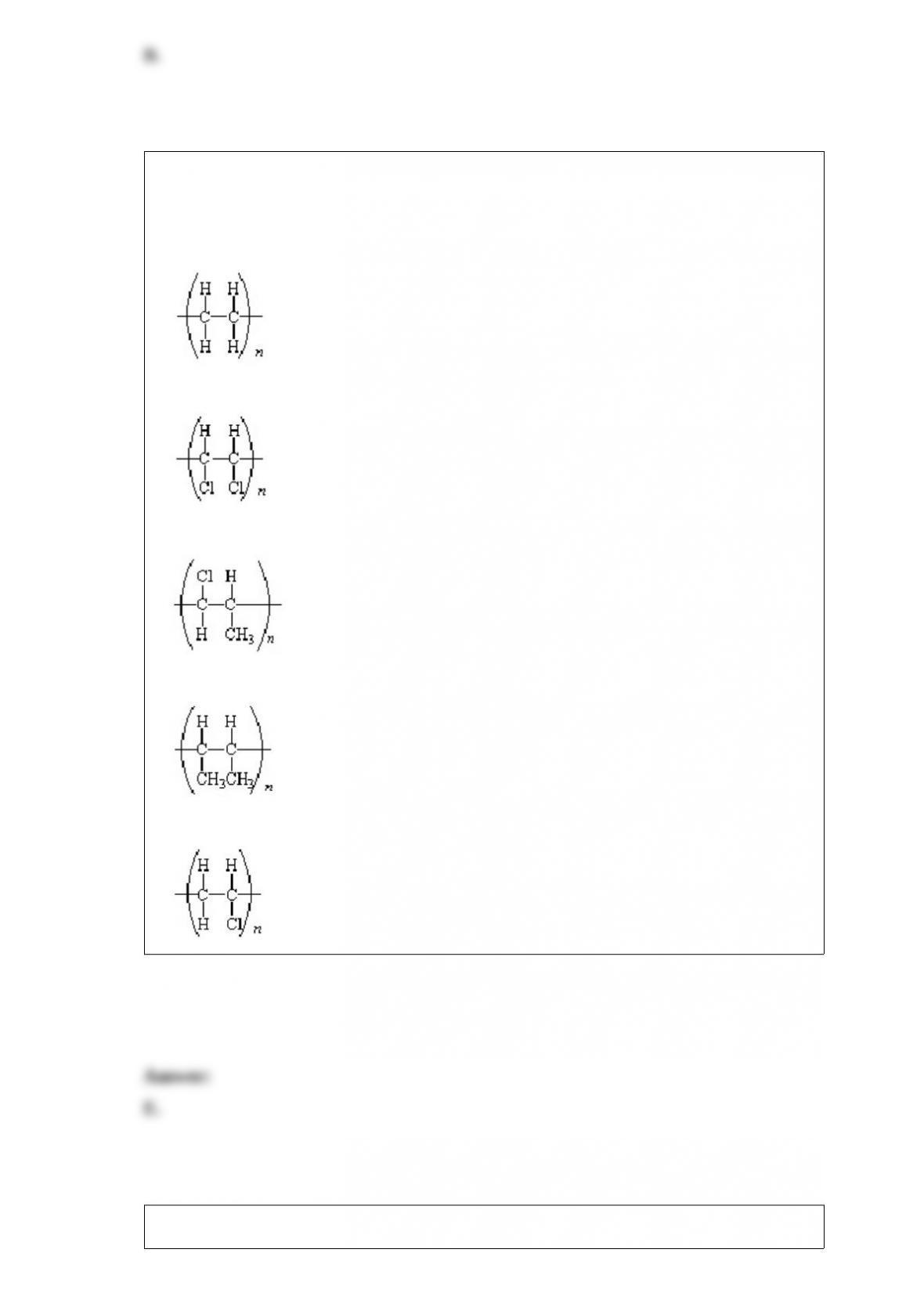
What is the equilibrium constant for the following reaction,
HCO2H(aq) + CN"(aq) HCO2
"(aq) + HCN(aq)
and does the reaction favor the formation of reactants or products? The acid
dissociation constant, Ka, for HCO2H is 1.8 10"4 and the acid dissociation constant for
HCN is 4.0 10"10.
A.K = 1.00. The reaction favors neither the formation of reactants nor products.
B.K = 2.2 10"6. The reaction favors the formation of products.
C.K = 2.2 10"6. The reaction favors the formation of reactants.
D.K = 4.5 105. The reaction favors the formation of products.
E.K = 4.5 105. The reaction favors the formation of reactants.
For a chemical system, DrG and DrG are equal when
A.the system is in equilibrium.
B.the reactants and products are in standard state conditions.
C.the equilibrium constant, K, equals 0.
D.the reaction quotient, Q, is less than 1.
E.the reactants and products are in the gas phase.