C. oxidation takes place alone, without an accompanying reduction.
D. oxidation occurs at the anode.
Alternative energy sources are currently being researched in effort to replace our
dependence on fossil fuels. Which is not a current research effort in this regard?
A. obtaining alternative fuels from renewable sources such as garbage
B. reintroducing the use of tetraethyl lead to increase the octane rating of gasoline
C. converting coal into gaseous and liquid fuels similar to petroleum products
D. increasing the use of farm product biomass, such as corn, to produce ethanol.
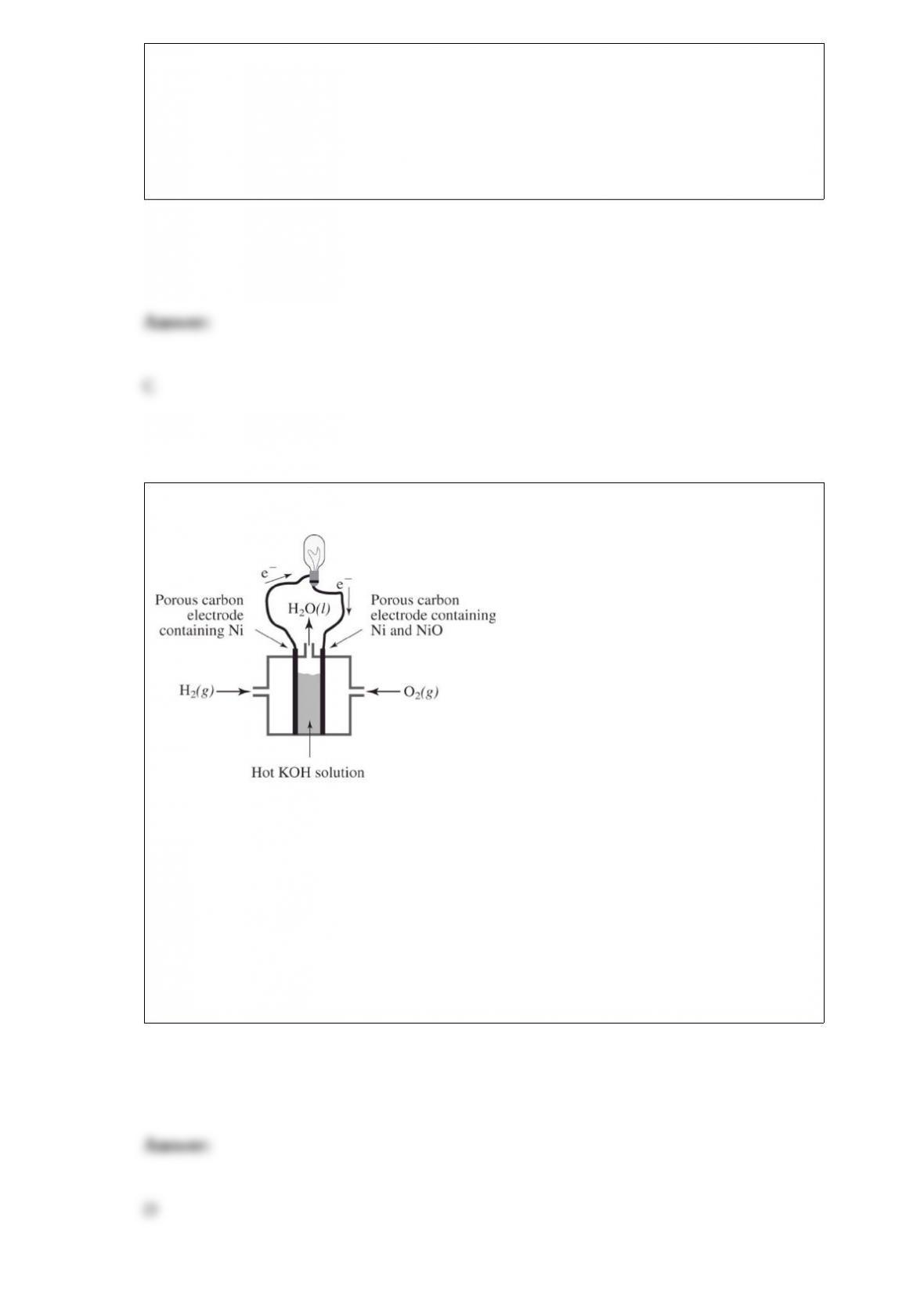
Which is not an advantage of using a solid electrolyte in a fuel cell?
A. It is impermeable to liquids and gases and will keep the fuel and oxidizer separated.
B. It cannot "dry out" and will allow the fuel cell to operate at higher temperatures than
one that uses water as part of the proton transfer mechanism.
C. It may use various fuels other than hydrogen gas, the fuel of choice in systems using
water as part of the proton transfer mechanism.
D. It eliminates the need for an oxidant; only a fuel is required.