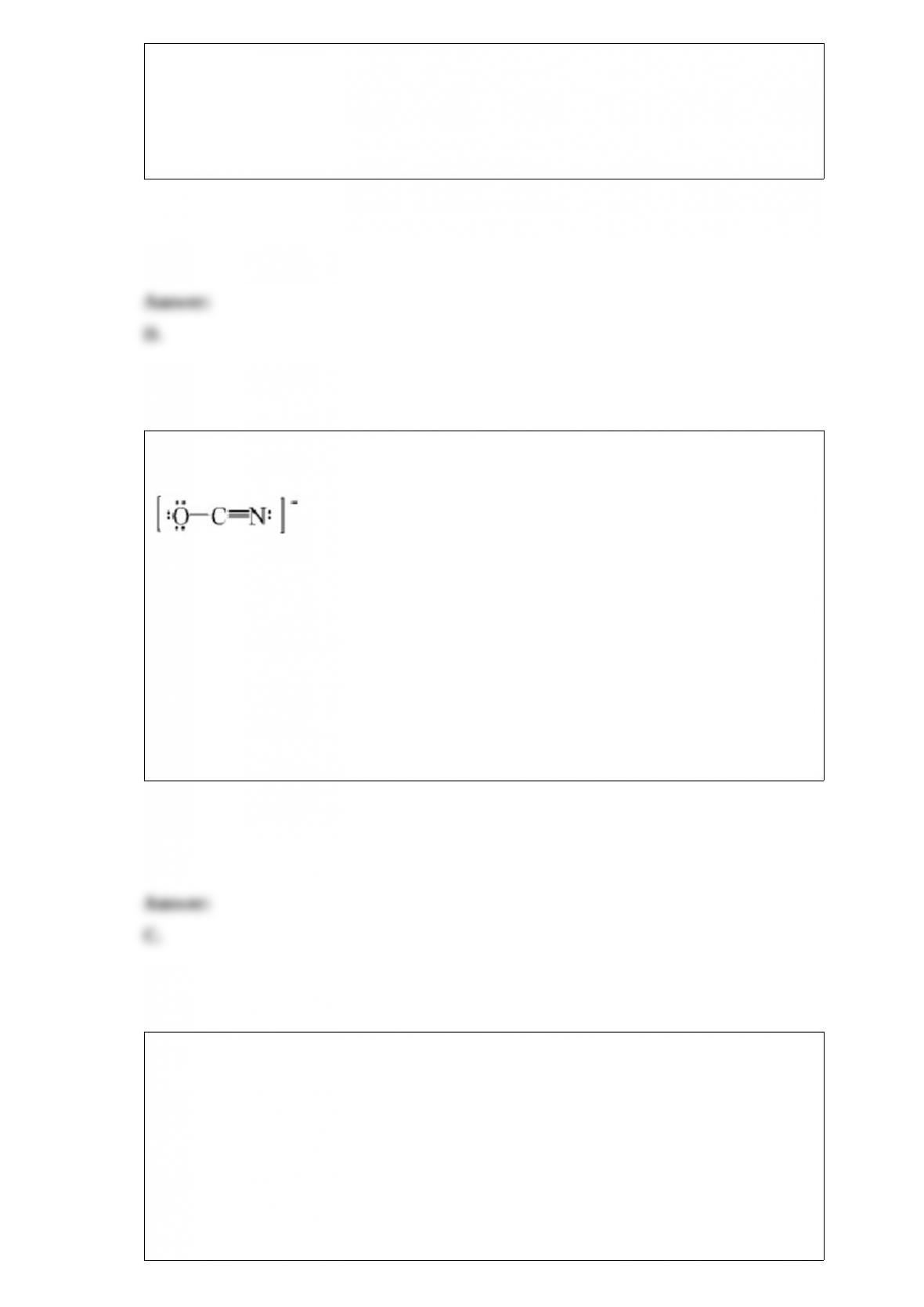
If an ionic compound with the formula MX forms a primitive cubic unit cell with the
anions (Xn") at the lattice points, the cations (M2n+) will occupy
A.all of the tetrahedral holes in each unit cell.
B.half of the tetrahedral holes in each unit cell.
C.the cubic hole in the center of the each unit cell.
D.the center of each face in each unit cell.
E.all of the octahedral holes in each unit cell.
As pure molecular solids, which of the following exhibits dipole-dipole intermolecular
forces: HBr, NBr3, SBr2, and CBr4?
A.Hbr only
B.CBr4 only
C.HBr and SBr2
D.NBr3 and CBr4
E.HBr, NBr3, and SBr2
Assume that an average and a standard deviation are calculated using a large number of
measurements. A single data point has a(n) ____ chance of falling within plus and